Question
Question: One mole of a monatomic ideal gas goes through a thermodynamic cycle, as shown in the volume versus ...
One mole of a monatomic ideal gas goes through a thermodynamic cycle, as shown in the volume versus temperature (V−T) diagram. The correct statement(s) is/are? [R is the gas constant]
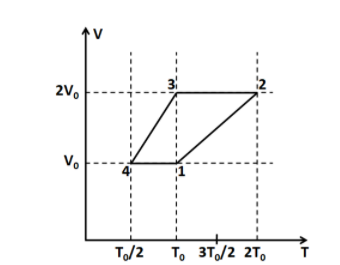
(A) Work done in this thermodynamic cycle (1→2→3→4→1) is ∣W∣=21RT0
(B) The ratio of heat transfer during processes 1→2 and 2→3 is Q2→3Q1→2=35
(C) The above the thermodynamic cycle exhibits only isobaric and adiabatic processes
(D) The ratio of heat transfer during processes 1→2 and 3→4 is Q3→4Q1→2=21
Solution
To solve this question, we need to identify each of the four processes in the given T-V diagram. Then we have to use the standard formulae for the work done and the heat transferred for the respective processes.
Complete step-by-step solution:
For process 1→2:
This process is represented by a straight line in the given V-T diagram. We know that the slope of a straight line is constant.
We know from the ideal gas equation that
PV=nRT
V=PnRT
Comparing with the equation of a line y=mx, we get the slope of the line representing the process 1→2 as
m=PnR
Since the slope is constant, so PnR is also a constant. Now, the quantity of the gas doesn’t change during the cycle, so the number of moles n remains constant. This means that in this process the pressure P is constant.
Now, substituting the values of the state
So the process 1→2 is an isobaric process.
For process 2→3:
This process is represented by a horizontal line in the given T-V diagram. So this means that the volume is constant in this process.
So the process 2→3 is an isochoric process.
Similarly we can show that the processes 3→4 and 4→1 are isobaric and isochoric, respectively.
Thus, the above thermodynamic cycle exhibits only isobaric and isochoric processes.
Hence, the option C is incorrect.
Now, we know that the work done in an isobaric process is given by
W=nRΔT
According to the question, we have one mole of gas. Therefore we have
W=RΔT.....................(1)
So the work done in the process 1→2 is given by
W1→2=R(T2−T1)
From the given diagram, T2=2T0, and T1=T0. Substituting these above we get
⇒ W1→2=RT0......................(2)
Also from (1) the work done in the process 3→4 is given by
W3→4=R(T4−T3)
From the given diagram, T3=T0, and T4=T0/2. Substituting these above we get
⇒ W1→2=−21RT0.......................(3)
Now, we know that the work done in an isochoric process is equal to zero. Therefore we have
⇒ W2→3=0..........................(4)
⇒ W4→1=0...............................(5)
The net work done in the complete cycle is
⇒ W=W1→2+W2→3+W3→4+W4→1
Putting (2) (3) (4) and (5) above, we get
⇒ W=RT0+0−21RT0+0
⇒W=21RT0
Thus, the work done in the complete cycle is ∣W∣=21RT0.
Hence, the option A is correct.
Now, we know that the heat transfer in an isobaric process is given by
⇒ Q=nCPΔT
Since there in one mole of gas, so we have
⇒ Q=CPΔT
So the heat transfer the process 1→2 is
⇒ Q1→2=CP(T2−T1)
Substituting T2=2T0, and T1=T0 we get
⇒ Q1→2=CPT0....................................(6)
Similarly the heat transfer in the process 3→4 is
Q3→4=−CP2T0..........................(7)
Also, we know that the work done in an isochoric process is given by
Q=nCvΔT
Since there in one mole of gas, so we have
Q=CvΔT
So the heat transfer the process 2→3 is
Q2→3=Cv(T3−T2)
Substituting T2=2T0, and T3=T0 we get
Q2→3=−CvT0 ………………………..(8)
Similarly, the heat transfer in the process 4→1 is
Q4→1=21CvT0 …….(9)
Dividing (6) by (8) we get
⇒ Q2→3Q1→2=−CvT0CPT0
⇒ Q2→3Q1→2=−CvCP
We know that CvCP=γ, and for a monatomic gas, we have γ=35. Therefore we have
⇒ Q2→3Q1→2=−35
Taking modulus both sides, we get
⇒ Q2→3Q1→2=35
Therefore, the option B is correct.
Now, dividing (6) by (7) we have
⇒ Q3→4Q1→2=−CpT0/2CPT0
Q3→4Q1→2=−2
Taking modulus both sides, we get
⇒ Q3→4Q1→2=2
Therefore, option D is incorrect.
Hence, the correct options are A and B.
Note: We could also attempt this question by obtaining the P-V diagram from the given T-S diagram. From there we could have directly got the value of the net work done in the cycle by calculating the area of the loop.
