Question
Question: One cyclic acetal form of D-galactose is shown. Which atom is the anomeric carbon? 
A.Atom A
B.Atom B
C.Atom C
D.Atom D
E.Atom E
F.Atom F
Solution
D - galactose is a type of carbohydrate molecule which is a monosaccharide sugar and serves as a source of energy. It is a C−4 epimer of glucose. It belongs to the class of organic compounds called hexoses. These are the monosaccharides in which the sugar unit has a six-carbon structure. When a molecule converts into its cyclic form it generates a new chiral center. The carbon atom that generates the new chiral center is called the anomeric carbon. The hemiacetal carbon atom becomes a new stereogenic center, commonly referred to as the anomeric carbon and the αand β isomers are known as anomers.
Complete step by step answer:
The straight-line structure of D- galactose is given as:
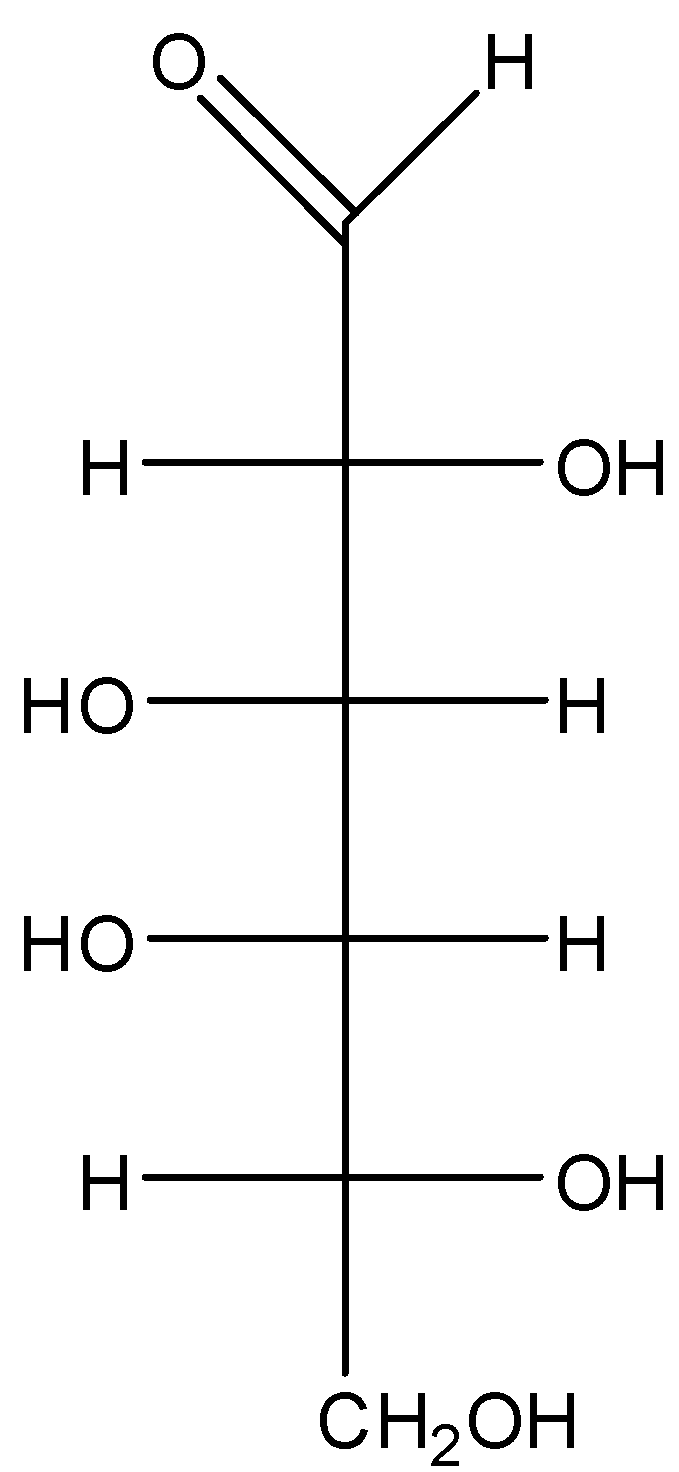
When we convert it into the cyclic structure it becomes:
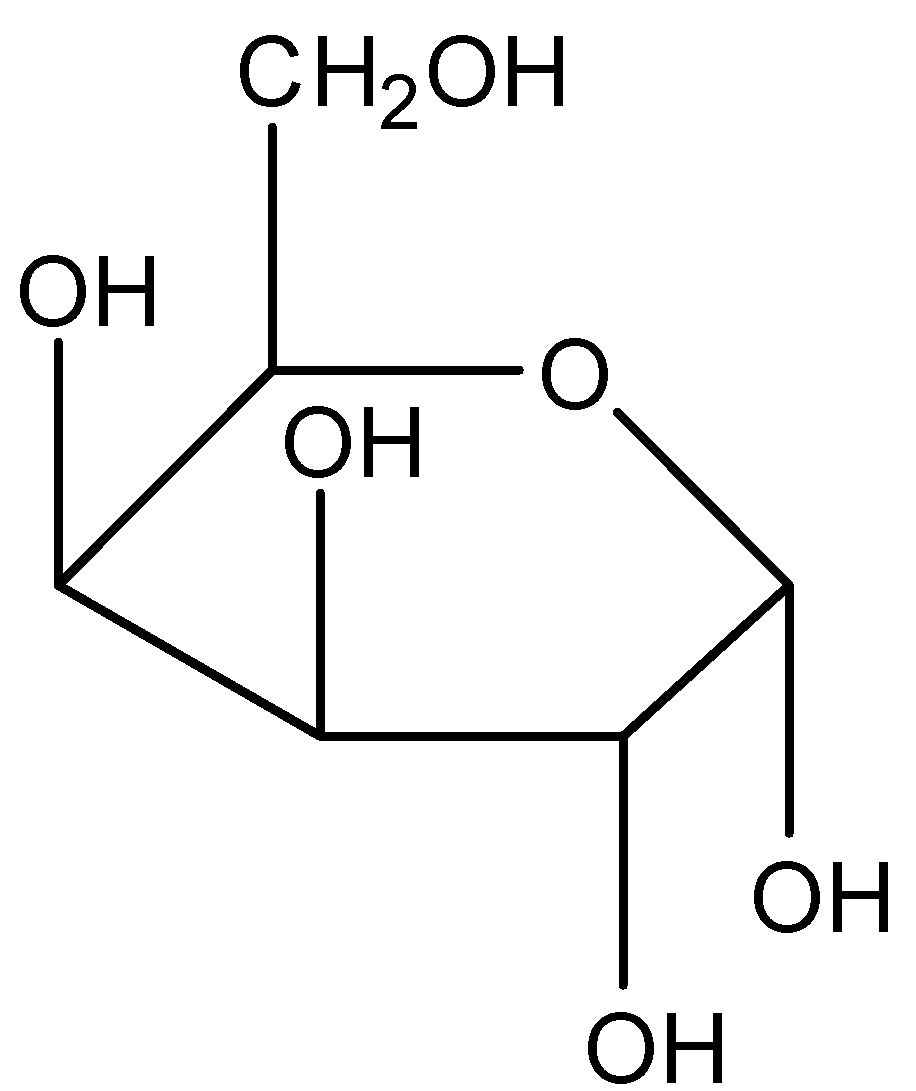
Now as we know that in the cyclic form of sugar the anomeric carbon is the carbon that was part of the carbonyl group in the straight-chain structure. When the chain converts into a ring, C−1 becomes the chiral center and hence C−1 is the anomeric carbon.
Hence option F (atom F) is the correct answer.
Note:
D- Galactose is an epimer of glucose hence the anomeric carbon of glucose and galactose will be the same.
The anomeric carbon in glucose and fructose is different as the anomeric carbon is the carbon that was part of the carbonyl group in the straight-chain structure. Thus the anomeric carbon in glucose is C−1 and in fructose, it is C−2.
The carbonyl group that is present in D-Galactose is an aldehydic group hence it is present on C−1 whereas in the case of fructose it is a ketonic group which cannot be present in a terminal position hence it is not present on C−1.
