Question
Question: On the hydrolysis of \({R_3}SiCl\), forms A.\({R_3}SiOH\) B.\({R_3}Si - O - Si{R_3}\) C.\({R_2...
On the hydrolysis of R3SiCl, forms
A.R3SiOH
B.R3Si−O−SiR3
C.R2Si=O
D.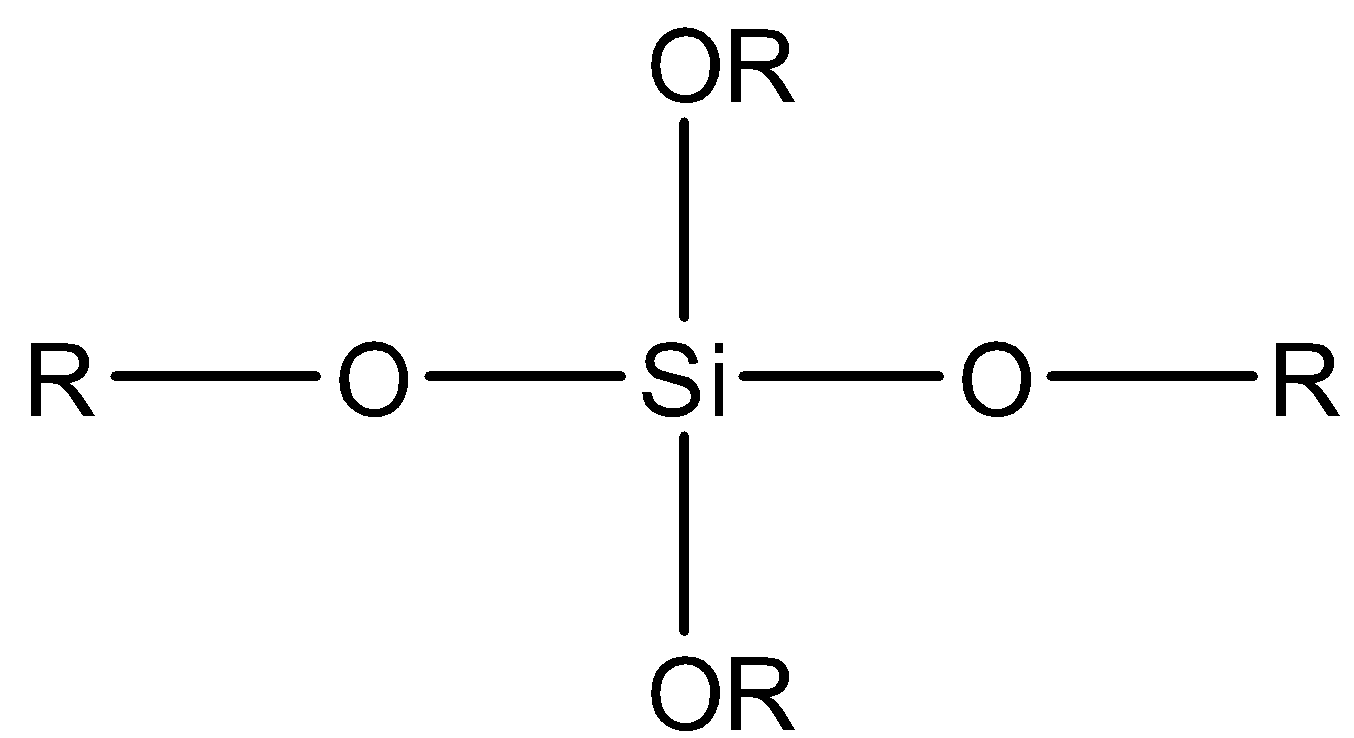
Solution
We can say a hydrolysis is the reaction in which a chemical bond present in the substrate is broken by its reaction with water. Usually, hydroxyl groups of water get linked to the electropositive atom and hydrogen present in water along with electronegative atoms forms a bond.
Complete step by step solution:
We know that hydrolysis reaction is a reaction in which a molecule of water breaks the bond in the substrate molecule. Now according to the question, R3SiCl is the substrate molecule.
Si−Cl bond is the most polar bond here. This is due to the highly electronegative element which is the chlorine. The electronegativity of silicon is similar to that of the carbon. So, we can say an atom of chlorine contains partial negative charge and a partial positive charge is present on silicon atom.
In water, OH− is the nucleophilic part and the electrophilic part is H+.
The mechanism could be given as,
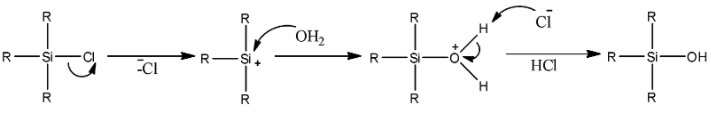
We can see that a cation is formed first. The cation gets nucleophilic attack by oxygen atoms of water. A silicon compound containing hydroxyl groups is formed. So, R3SiOH is the final product.
We can write the chemical reaction as,
R3SiClH2OR3SiOH+HCl
R3SiOH+R3SiOHCondensation−H2OR3Si−O−SiR3
The hydrolysis product of R3SiCl is R3SiOH. Option (1) is correct.
When we condense the product R3SiOH, the product formed will be R3Si−O−SiR3 and molecules of water. Option (2) is also correct.
So, we can say R3SiCl on hydrolysis reaction forms R3SiOH.
And hence option 1 and 2 is correct.
Note: We have to remember that the common formula of aryl (or) acyl substituted silicon chlorides are RnSiCl4−n and they are known as silicones. We can use silicones as electrical insulators, sealants, and in the manufacture of grease and waterproofing fabrics.
