Question
Question: On reductive ozonolysis, what is the product? 
Solution
We need to realize that ozonolysis is portrayed as a sort of cyclo expansion that breaks bonds. In this response, cleavage of the pi bond will occur so that the end results give two carbonyl gatherings.
Complete answer:
In the main response 1,3 -dipolar cyclo expansion response will occur. The reagent is ozone. Ozone is an even twisted particle with a focal decidedly charged oxygen iota and two terminal oxygen iotas that share a negative charge. It's anything but a 1,3 -dipole and does regular 1,3 -dipolar cyclo increases with alkenes. The item is a truly insecure compound. The O−O single bond is a powerless bond a lot more fragile than the N−O bond. We have been portrayed as powerless in past models, and this hetero cycle has two of them. It quickly decays by an opposite 1,3 -dipolar cyclo expansion.
The items are a straightforward aldehyde on the left and another, fairly unsteady looking atom 1,3 -dipole known as a carbonyl oxide on the right. At any rate it no longer has any obvious O−O single securities. Being a 1,3 -dipole, it presently adds to the aldehyde in a third cyclo addition step. It may very well add back the manner in which it came, yet it very much wants to include the alternate path round with the nucleophilic oxyanion assaulting the carbon iota of the carbonyl gathering.
The item is ozonide, which isn't steady, the ozonide is hazardous in nature. Thus, it promptly deteriorates by dimethyl sulfide to yield atoms of aldehyde. The reaction is given below,
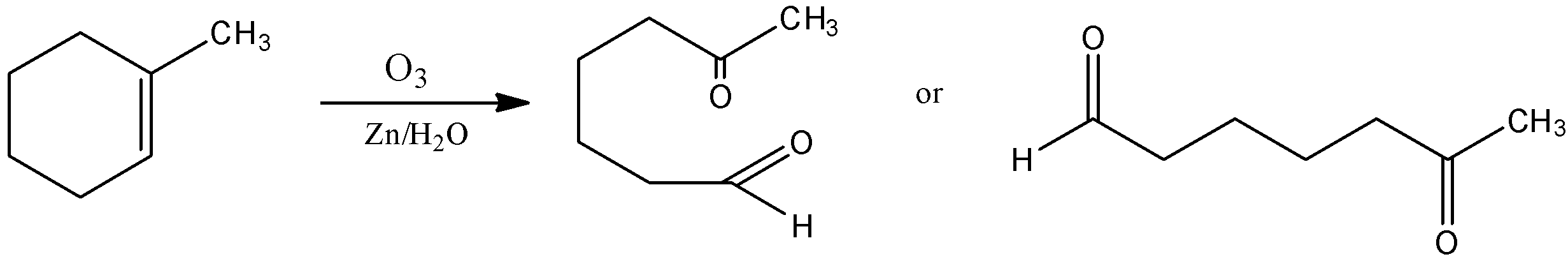
Note:
Ozonolysis is a strategy for oxidatively cutting alkenes or alkynes utilizing ozone, a receptive allotrope of oxygen. The interaction takes into consideration carbon-carbon twofold or triple bonds to be supplanted by twofold bonds with oxygen. This response is frequently used to recognize the construction of obscure alkenes.
