Question
Question: On ozonolysis, ethylene gives: A. aldehyde B. ketone C. carboxylic acid D. ether...
On ozonolysis, ethylene gives:
A. aldehyde
B. ketone
C. carboxylic acid
D. ether
Solution
Ozonolysis is the oxidative cleavage of carbon-carbon double bond using ozone as an oxidizing agent is called ozonolysis. The reaction is performed in organic solvents like dichloromethane, methanol or acetone at −78∘C.
Complete step by step answer:
Ozone is the molecule containing three atoms of oxygen. Ozonolysis of ethylene or ethane involves the cleavage of the double bonds in the alkenes using ozone molecules (O3). Ozone is very reactive. Ozone acts as an oxidizing agent.
When ozone, in any of the solvents, is reacted with ethane, it forms an intermediate called ozonide molecule. Initially the electrophile of ozone is added to the carbon-carbon double bond which forms an intermediate. Then it breaks and forms carbonyl and carbonyl oxide molecules. This rearranges and makes a stable ozonide intermediate. This is followed by workup results in the formation of respective products. Thus it converts to corresponding carbonyl compounds. The reaction is given below:
C2H4+O3⇄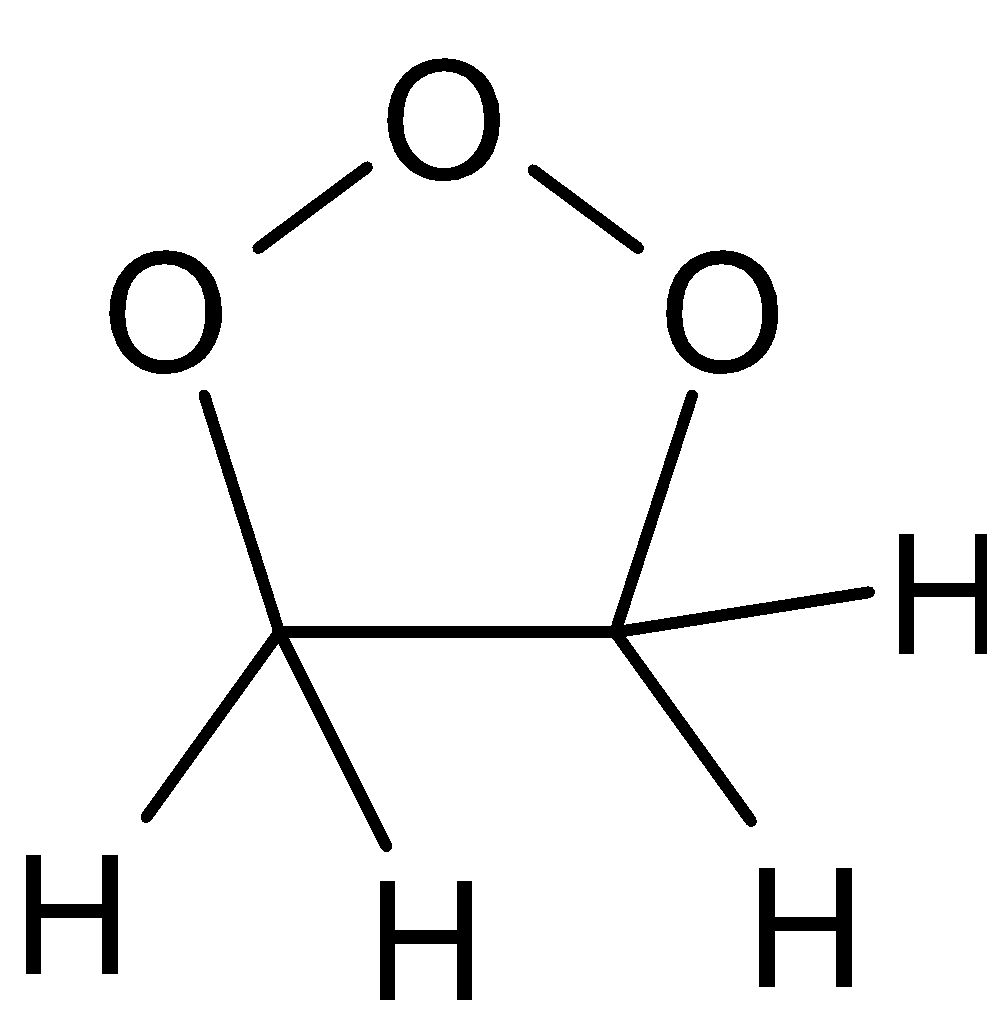 ⇄
⇄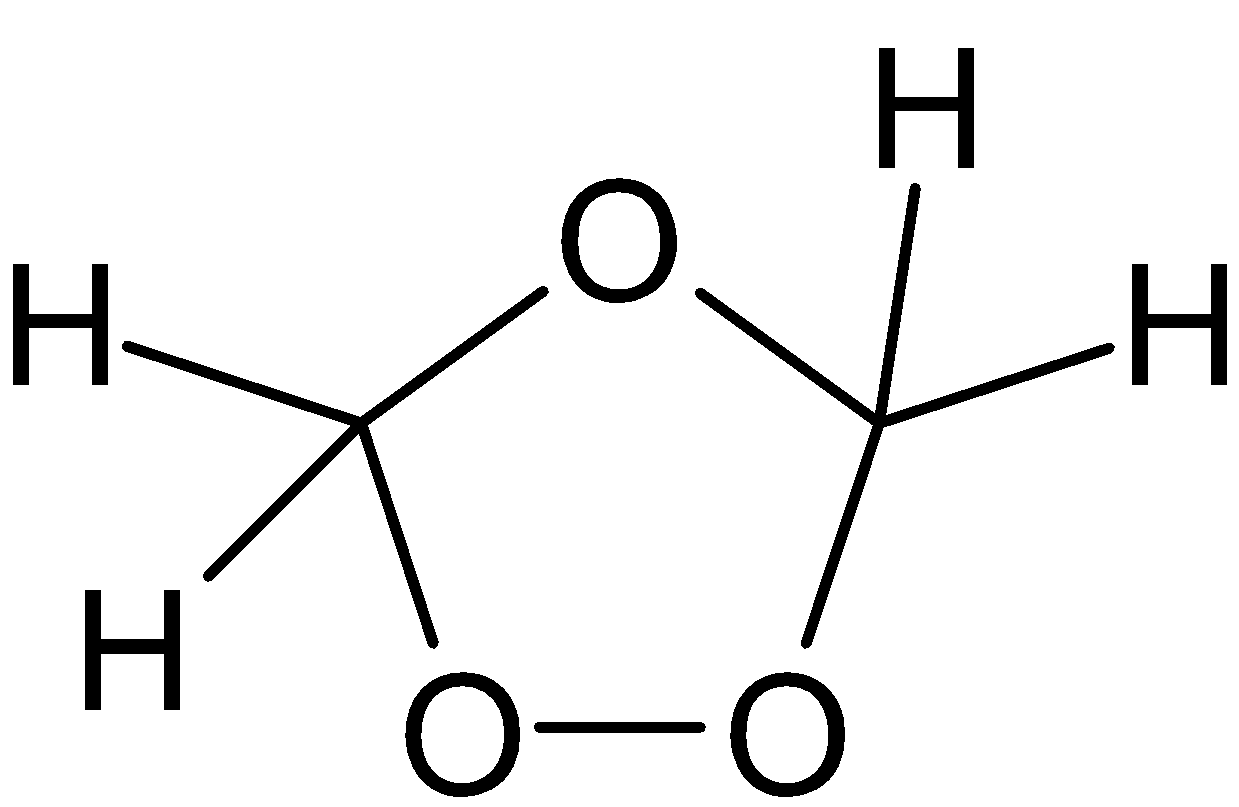 →HCHO
→HCHO
Hence aldehydes are formed.
Workup is divided into three types:
-Using mild reducing agents like Me2S or PPh3 or zinc dust
It produces carbonyl compounds, particularly aldehyde or ketone.
-Strong reducing agents like LiAlH4 or NaBH4.
It produces alcohol.
-Oxidizing agents like H2O2 or O2.
It produces carboxylic acids.
Hence option A is correct.
Note:
Ozone is an allotrope of oxygen. It breaks both σ and π bonds of alkene. Oxidizing or reducing agents are added to give corresponding carbonyl compounds. Alkynes may produce different types of acids on ozonolysis.
