Question
Question: On oxidation with alkaline \(KMn{O_4}\) , \('X'\) compound gives \({C_3}{H_6}{O_2}\) and \({C_2}{H_4...
On oxidation with alkaline KMnO4 , ′X′ compound gives C3H6O2 and C2H4O2 as two carboxylic acids. What would be the IUPAC name of alkyne ′X′?
A.But-2-yne
B.But-1-yne
C.Pent-2-yne
D.Hex-1-yne
Solution
The best way to solve the conversion problems is to follow the reaction step by step. We will focus on the properties of reagent. For example: if the reaction is with an oxidizing agent it will give the oxidized products. The product formed also helps in detecting the reactant.
Complete step by step answer:
First, we will write a reaction in terms of products and reactants. Here ′X′ on oxidation gives two carboxylic acids as C3H6O2 andC2H4O2.
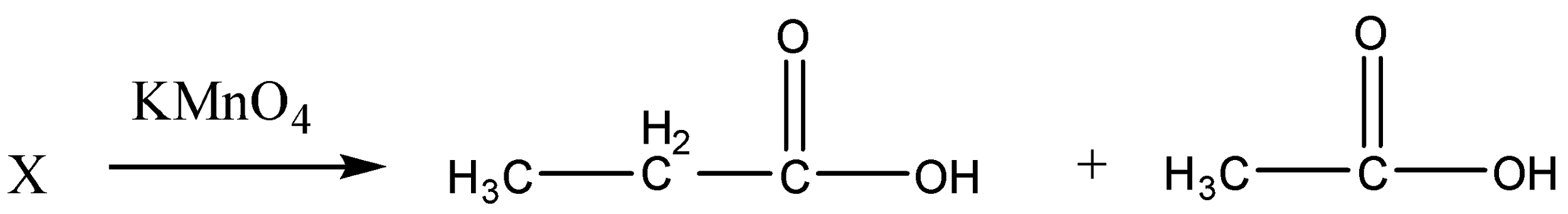
So the reaction given above shows that an alkyne ′X′ on oxidation gives two carboxylic acids C3H6O2 and C2H4O2 which is the only possible structure of these given carboxylic acids.
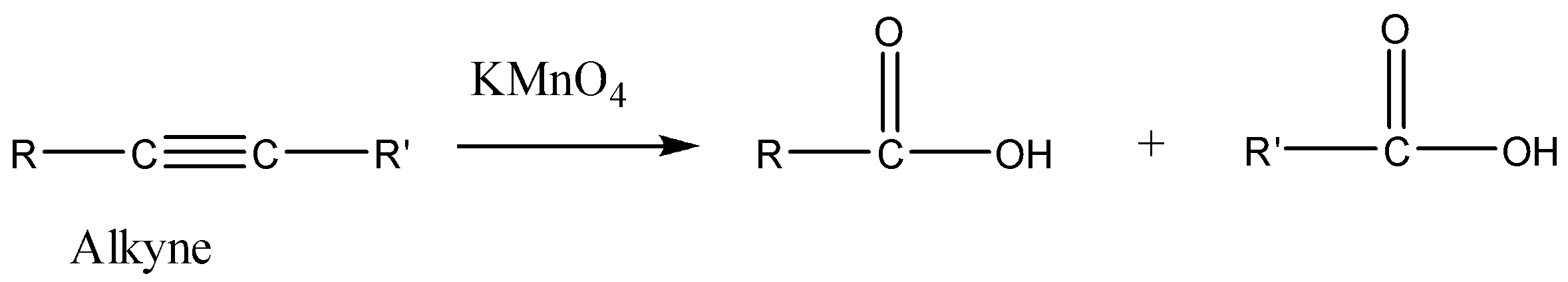
So now we need to find the alkyne ′X′. We know that oxidation with alkaline KMnO4 alkyne gives carboxylic acids. We can understand it with the help of the reaction given below.
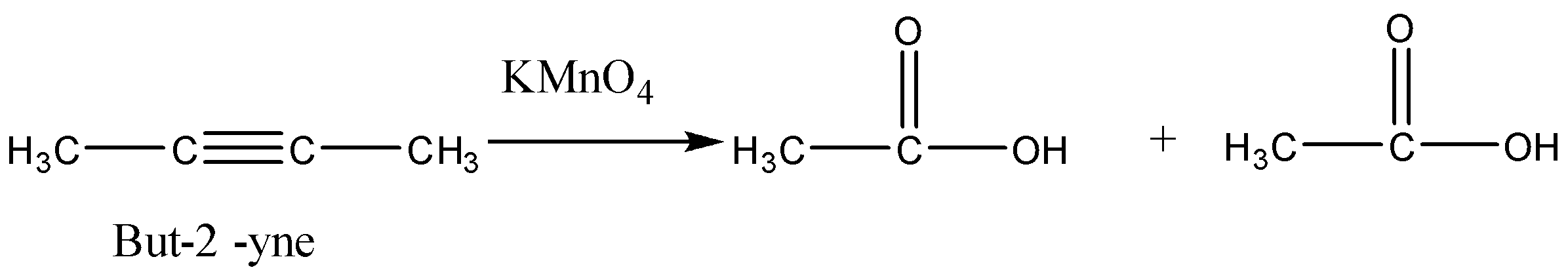
So we will check the options one by one to get the desired product. So consider option (A) But-2-yne. This reaction is not giving us the given carboxylic acids.
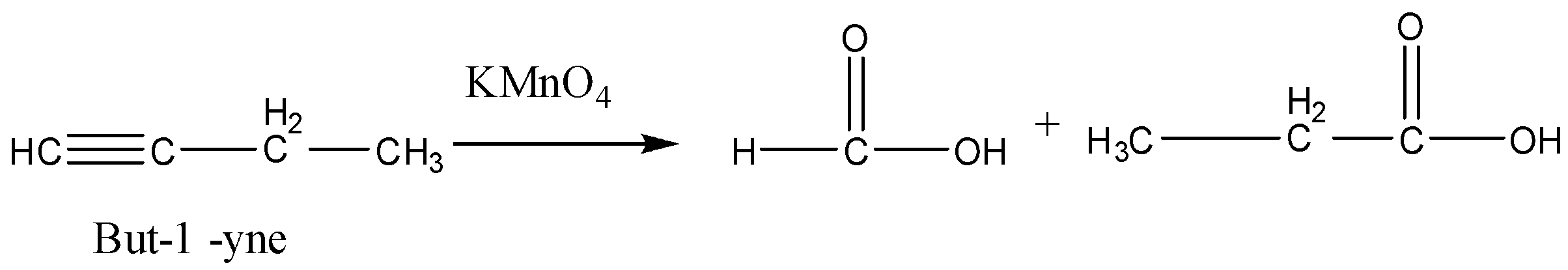
Now we will consider option (B) But-1-yne. This alkyne will also not give the required carboxylic acids.
Now we will consider option (C) Pent-2-yne. This alkyne will give us the carboxylic acids given in the question.
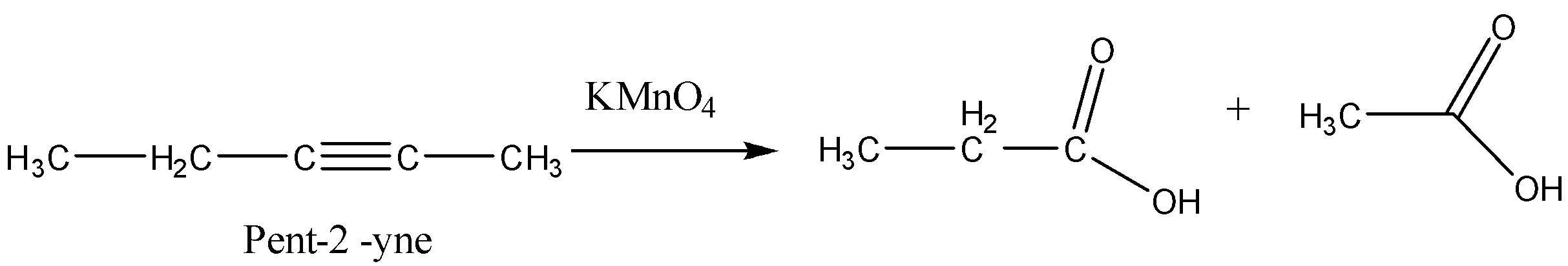
Now the last option (D) Hex-1-yne. It will also give different carboxylic acids.
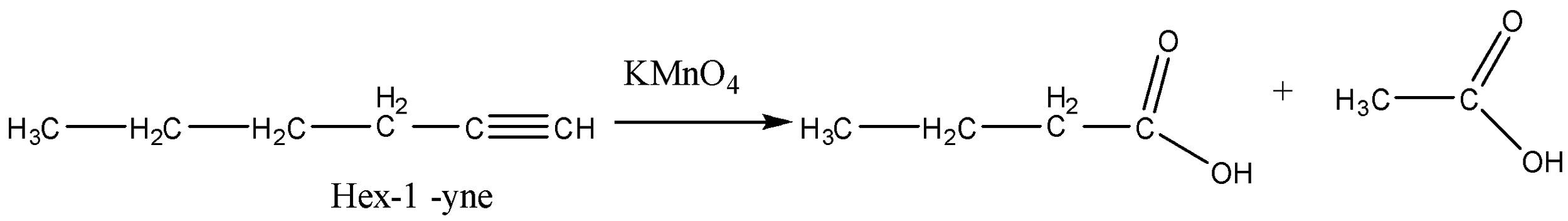
So from the above reactions, we can conclude that ′X′ is Pent-2-yne.
Therefore, option (C) is correct.
Note:
Alkaline KMnO4 is a powerful oxidizing agent. The oxidation state of Mn is +7. It is the highest oxidation state of manganese.
Terminal alkyne is the alkyne in which the carbon-carbon triple bond is at last.
