Question
Question: On monochlorination of \(2 - methylbutane\), the total number of chiral compounds formed is, \( ...
On monochlorination of 2−methylbutane, the total number of chiral compounds formed is,
A.2B.4C.6D.8
Solution
We know that a molecule or ion is called chiral only when there is no superimposable mirror image and there is no plane of symmetry in the molecule. This property is known as chirality and the compounds with the same molecular formula but differ in the arrangement of the atom are said to be isomers. The compounds which are mirror images but are not identical; to each other are called enantiomers.
Complete step by step answer:
Monochlorination of 2−methylbutane forms four isomers,
The structure of 4 isomers is,
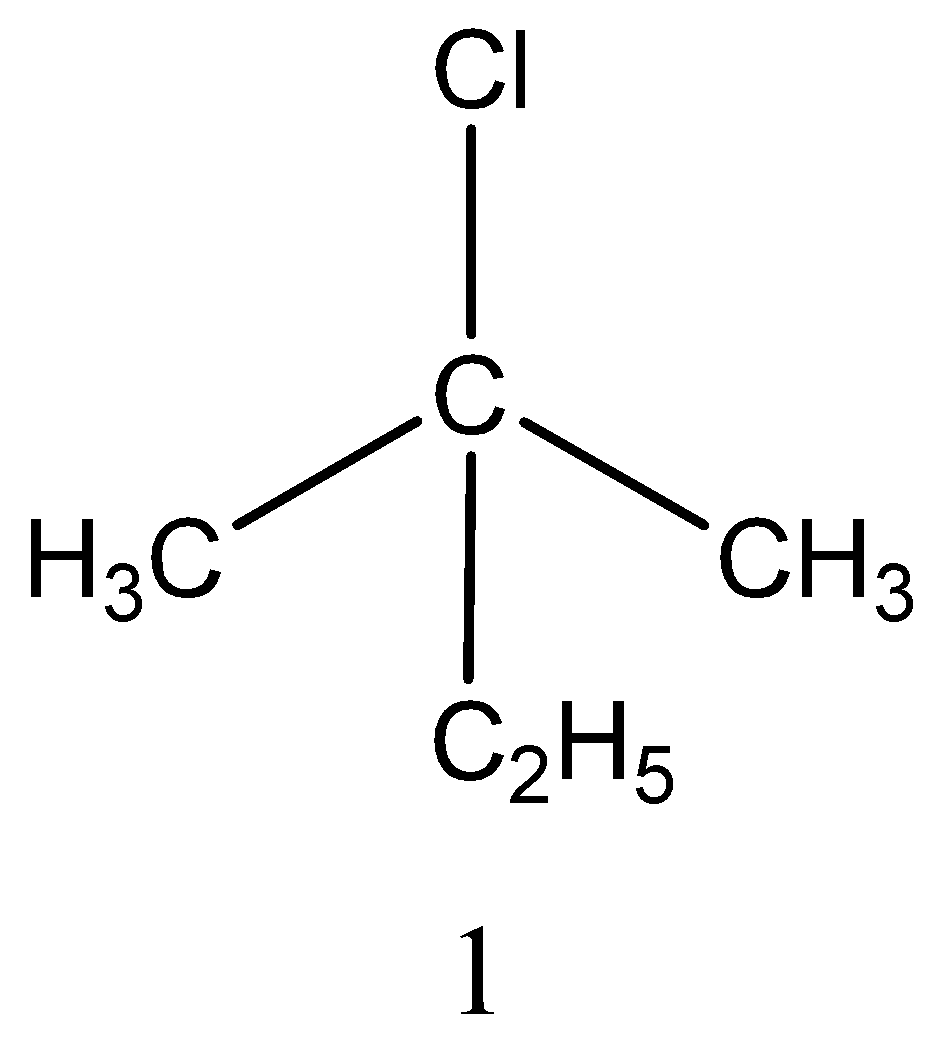
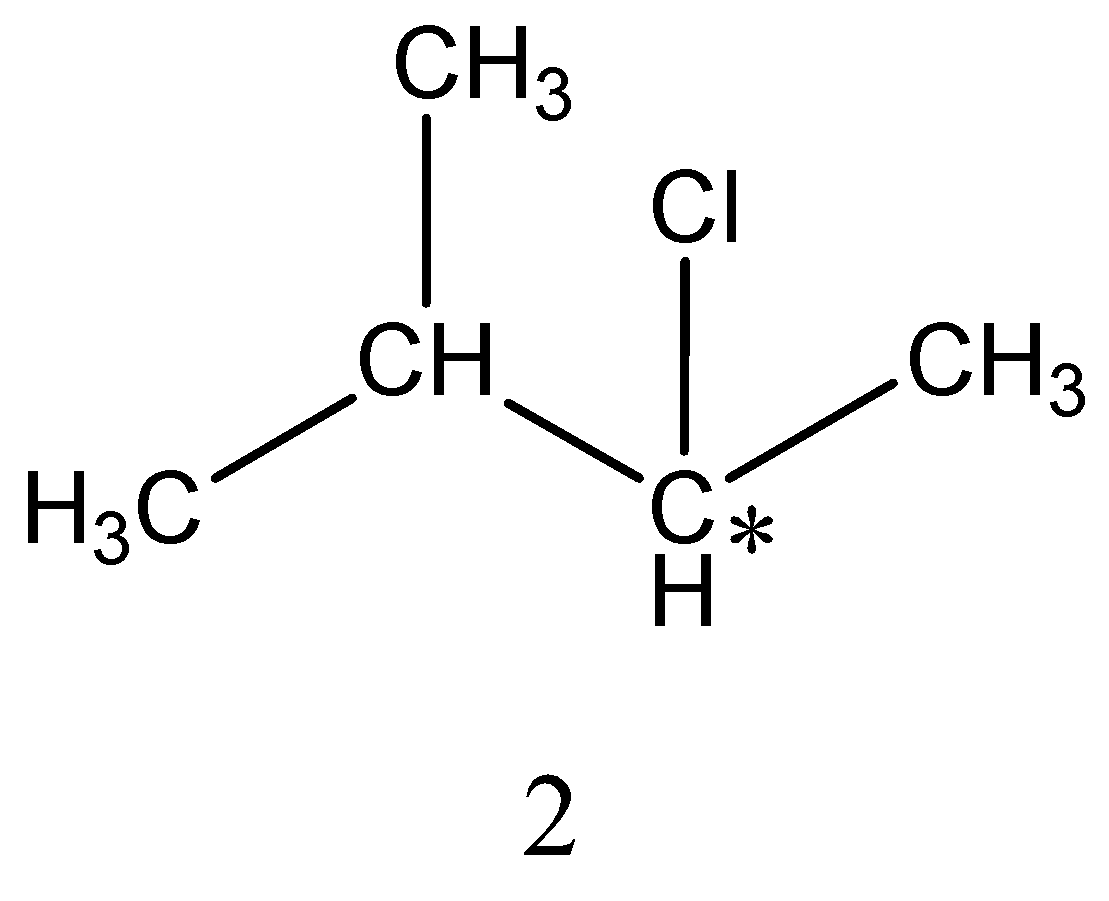
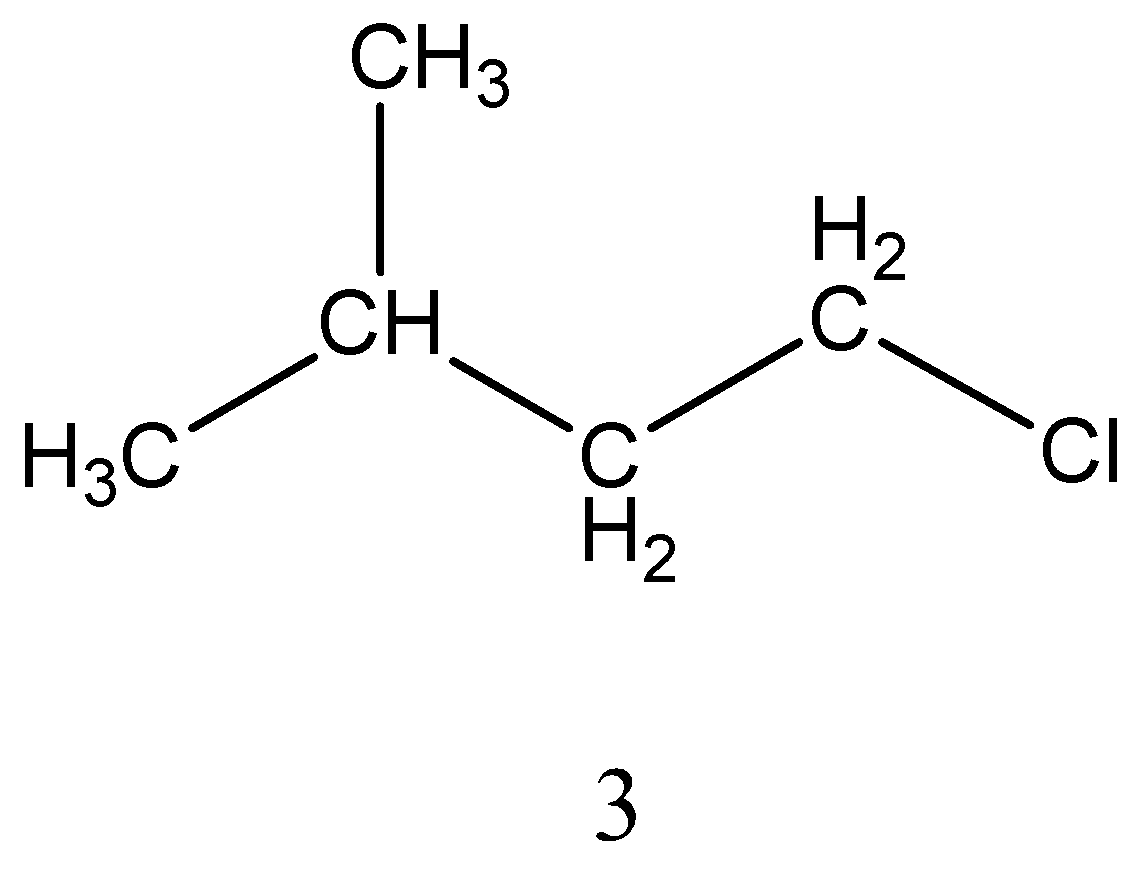
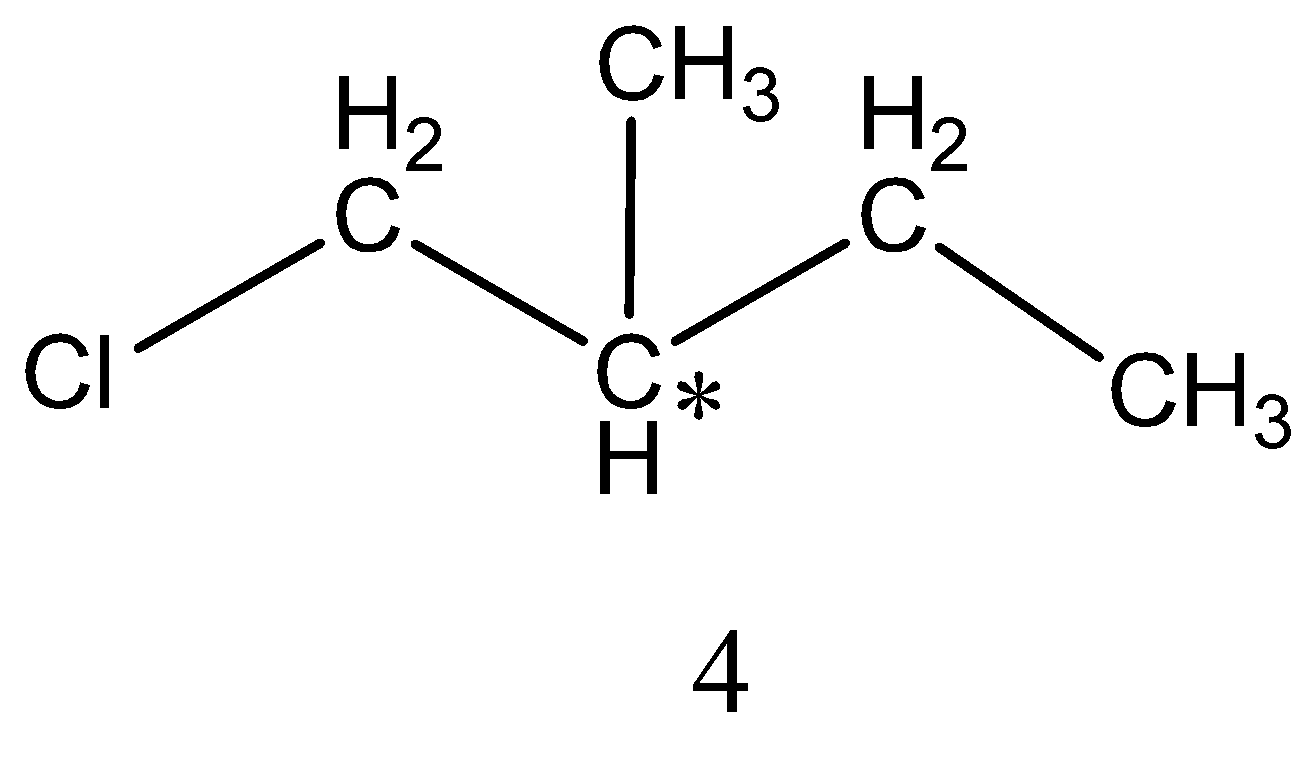
Among the four isomers only two isomers are chiral. As we already know that if carbon is attached to four different groups then it is referred to as chiral carbon. Thus, among the four isomers 2&4 has chiral carbon.
Therefore, the option A is correct.
Note:
Enantiomers versus Chiral:
Chiral recognition is often described because of the inequity between the 2 enantiomers of a chiral molecule. Because the physical properties that are typically used to separate molecular species are identical within the case of enantiomers, it's difficult to separate the 2 species. It’s only through the interactions with a discriminating secondary species that physical differences are often observed. The structural basis of enantiomerism is named chirality. Enantiomers are in every other respect chemically identical. A pair of enantiomers is distinguished by the direction during which when dissolved in solution they rotate polarized light either Dextro or levo rotatory, hence the term optical isomers. When two enantiomers are present in equal proportions they're collectively mentioned as a racemic mixture that doesn't rotate polarized light because the optical activity of every enantiomer is cancelled by the opposite .
