Question
Question: On heating \[{{K}_{4}}Fe{{(CN)}_{6}}\] with conc.\[{{H}_{2}}S{{O}_{4}}\] gives the gas: A.\[S{{O}_...
On heating K4Fe(CN)6 with conc.H2SO4 gives the gas:
A.SO2
B.CO2
C.CO
D.NO2
Solution
Hint: The gas produced on heating K4Fe(CN)6 with conc.H2SO4 has unsaturated carbon-oxygen bond with two pi bonds and one sigma bond. In coordination complexes, it participates in back-bonding with the metal atom and known as carbonyl ligand.
It is very toxic to the human body.
Complete step by step answer:
The gas produced on heating K4Fe(CN)6 with conc.H2SO4 is carbon monoxide. Its chemical formula is CO.
The structure of COis as follows:
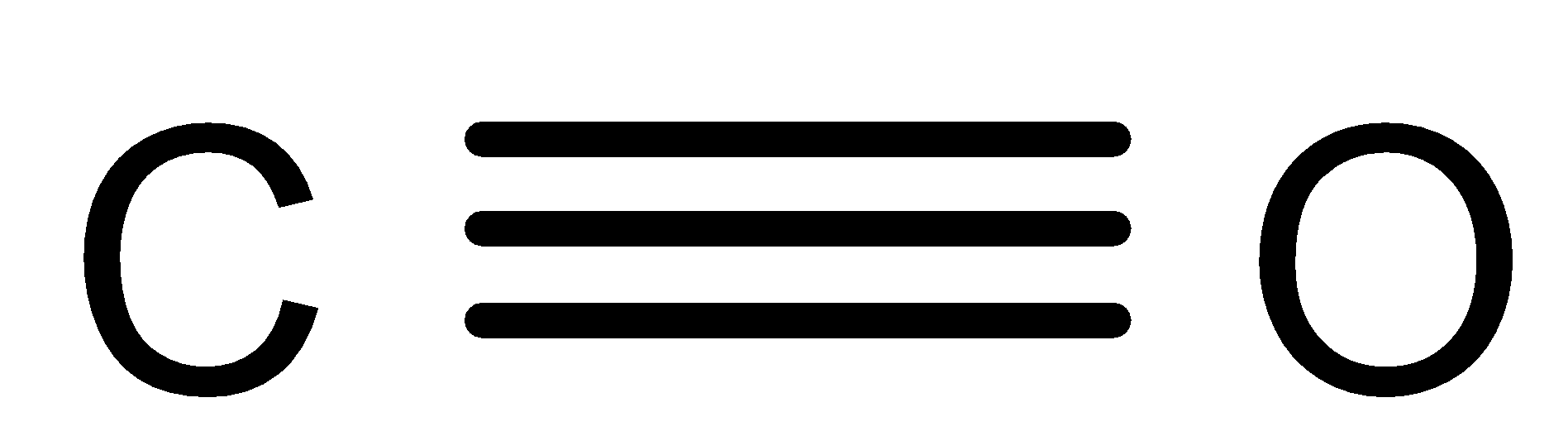
It is produced from the partial oxidation of the carbon-containing compounds.
It is a gas without any colour, taste and odour. It is a flammable gas and produces blue flame on heating. It is slightly lighter than air and is also known as ‘carbonous oxide’.
On reaction with water, K4Fe(CN)6dissociates as:
[Fe(CN)6]4−⇌ Fe+2(aq) + 6CN−(aq)
When, Fe+2reacts with conc.H2SO4, it dissociates into Fe+2(aq), HCN andSO42−(aq) ions.
Finally, after treating it with the acidic hydrogen followed by dehydration, the products formed are carbon monoxide (CO), ferrous sulphate (FeSO4) and other side products.
The reaction is as follows:
K4[Fe(CN)6] +6H2SO4 +6H2O → 6CO + FeSO4 +3(NH4)2SO4 + 2K2SO4
So, on heating K4Fe(CN)6 with conc.H2SO4, the gas produced is carbon monoxide.
So, the correct option is C.
Note: On heating K4Fe(CN)6 with diluteH2SO4, the gas evolved is hydrogen cyanide (HCN) in the intermediary step.
It is an indirect and weak greenhouse gas. Its emission in the atmosphere causes global warming. When it enters our body, it deprives the vital organs of the body from oxygen and hence causing fatal problems in the human body.
