Question
Question: On heating glycerol with excess amount of \({\rm{HI}}\), the product formed is: A. Allyl iodide ...
On heating glycerol with excess amount of HI, the product formed is:
A. Allyl iodide
B. Isopropyl iodide
C. Propylene
D. 1,2,3−triiodopropane
Solution
Glycerol has three hydroxyl groups that take part in the reaction and the final product is formed after going through some intermediates. These intermediates decide which type of reaction is going to happen.
Step by step answer: We know that one method of preparing halogenated alkanes is the reaction of alcohols with suitable hydrogen halide. However, we have to take into account the reactant’s properties and reaction conditions as well while deducing the final product. Here, we are given a reaction of heating glycerol with an excess amount of HI. Let’s look at our reactant, first of all which has the following structure:
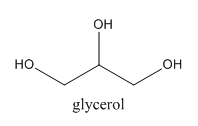
As we can see, three −OH groups are present at each of the three carbon atoms. We can write its proper name to be Propane−1,2,3−triol. Now, upon reacting with excess of HI, we can say that all of the three −OH groups would be replaced by iodides as in any normal reaction but here, this product is not stable but an intermediate one. We can show the reaction as follows:
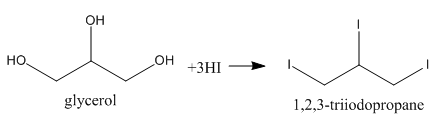
We can write the next step of the reaction in which 1,2,3−triiodopropane loses a molecule of iodine as follows:
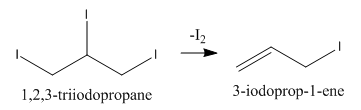
We know that 3−iodoprop−1−ene is also known as allyl iodide and it can add one molecule of HI as follows:
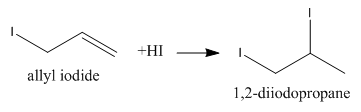
As we can see that this addition product, 1,2−diiodopropane can also eliminate one iodine molecule as follows:
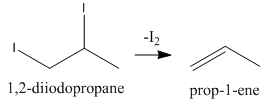
Now, again we have an alkene that can add one molecule of HI to give the final product as follows:
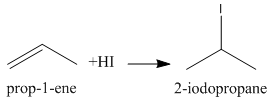
We know that 2−iodopropane is also known as Isopropyl iodide.
Hence, the correct option is B.
Note: Here, we have used both the common names as well as IUPAC names for the compounds so it is necessary to know both systems. We have to look for the reaction condition very carefully because the above reaction is possible only when we have an excess amount of HI.
