Question
Question: on find the number moles of 1) 3.011 x $10^{23}$ molecules of $CaCO_3$ 2) 1.5055 x $10^{22}$ molecul...
on find the number moles of
- 3.011 x 1023 molecules of CaCO3
- 1.5055 x 1022 molecules of H2SO4
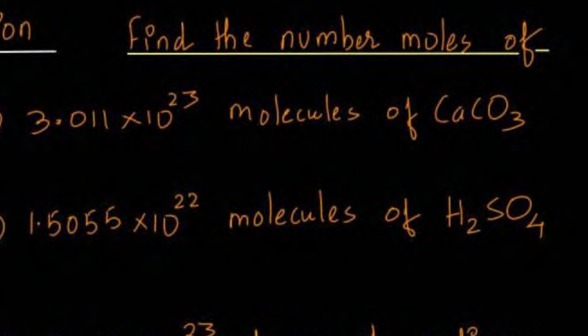
- 0.5 moles of CaCO3
- 0.025 moles of H2SO4
Solution
To find the number of moles from a given number of molecules, we use Avogadro's number (NA), which states that one mole of any substance contains 6.022×1023 particles (molecules, atoms, ions, etc.).
The formula to calculate the number of moles is: Number of moles=Avogadro’s number(NA)Given number of molecules Where NA=6.022×1023 molecules/mol.
1) For 3.011 x 1023 molecules of CaCO3:
Number of moles=6.022×1023 molecules/mol3.011×1023 molecules Number of moles=6.0223.011 mol Number of moles=0.5 mol
2) For 1.5055 x 1022 molecules of H2SO4:
Number of moles=6.022×1023 molecules/mol1.5055×1022 molecules Number of moles=6.0221.5055×10231022 mol Number of moles=0.25×10−1 mol Number of moles=0.025 mol
Explanation of the solution:
The number of moles is calculated by dividing the given number of molecules by Avogadro's number (6.022×1023 molecules/mol).
- For 3.011×1023 molecules of CaCO3: (3.011×1023)/(6.022×1023)=0.5 moles.
- For 1.5055×1022 molecules of H2SO4: (1.5055×1022)/(6.022×1023)=0.025 moles.
