Question
Question: On an isothermal process, there are two points A and B at which pressures and volumes are \[\left( {...
On an isothermal process, there are two points A and B at which pressures and volumes are (2P0,V0) and (2P0,V0) respectively. If A and B are connected by a straight line, find the pressure at a POINT on this straight line at which temperature is maximum
A 34P0
B 35P0
C 23P0
D 57P0
Solution
This question is based on a thermodynamic process called isothermal process. We have to know about the isothermal process. In this process, the temperature throughout the thermodynamic process remains constant. We use the isothermal expression and P-V diagram to find the pressure and volume at which the temperature is maximum.
Complete step by step answer:
We know that the isothermal is the contact temperature process. So ,only changes occur in volume and pressure throughout the process.
Now, consider the P-V diagram on which two points A and B lie on it.
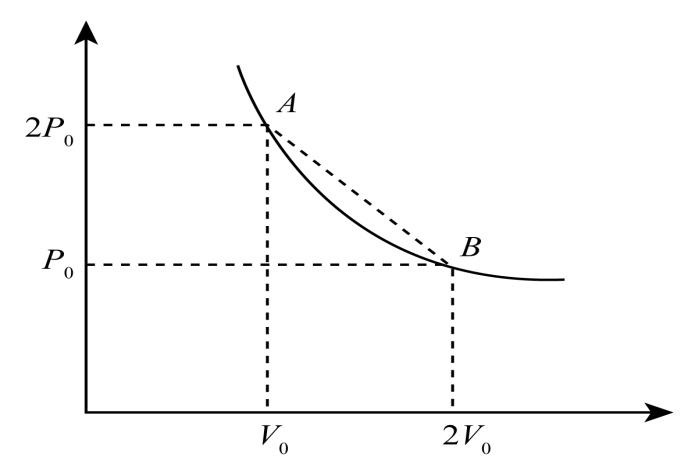
Here, in point A the pressure is 2P0 and volume is v0 and in point B, the pressure is P0 and volume is 2v0. We have to find the pressure on a straight line where the temperature is maximum.
From the given diagram, there is a straight line that follows the straight line equation y=mx+c.
Therefore, it can written as,
P=mv+c........(i)
Here, m is the slope.
Now, we have to calculate the slope of the line.
Therefore,
m=−(2V0−V02P0−P0)
Now, substitute the value in equation (i) we get,
P=(2V0−V02P0−P0)v+c ⟹P=−V0P0×V+c............(ii)
We have to satisfy the equation by substitute the value (2P0,V0) we get,
2P0=−V0P0×V0+c ⟹c=3P0
Substitute this value in equation (ii) we get,
P=−V0P0×V+3P0 ⟹V0P+P0V=3P0V0.......(iii)
Now, by using the ideal gas equation,
PV=nRT ⟹V=PnRT
Substitute this value in equation (iii) we get,
V0P+P0(PnRT)=3P0V0 ⟹T=P0nR3P0V0P−V0P2........(iv)
It is given in question that we have to find the pressure at maximum temperature.
Therefore,
dPdT=0 ⟹P0nR3P0V0−2V0P=0 ∴P=23P0
So, the correct answer is “Option C”.
Note:
In this question, we have to know the isothermal process. And with the help of P-V diagram we can find the maximum temperature. Using symmetry, the maximum temperature is at the midpoint of the point A and B.
