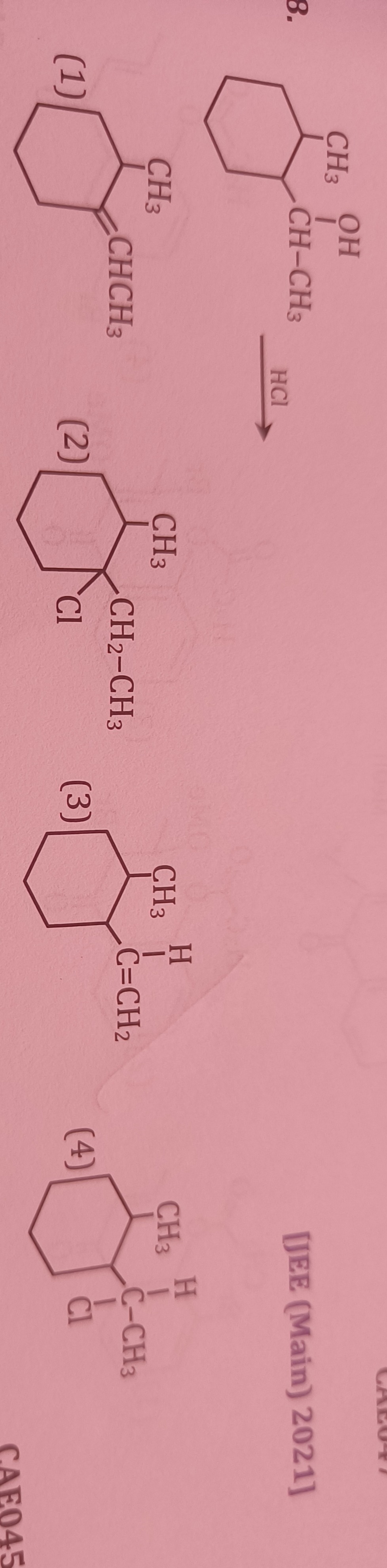
Question
Question: OH CH3 8. CH-CH3 HCI (1) CH3 CHCH3 CH2-CH3 (2) Cl [JEE (Main) 2021]...
OH CH3 8.
CH-CH3 HCI (1) CH3 CHCH3
CH2-CH3 (2) Cl [JEE (Main) 2021]
A
(1)
B
(2)
C
(3)
D
(4)
Answer
1
Explanation
Solution
The reaction proceeds via an SN1 mechanism. The secondary alcohol is protonated, followed by the loss of water to form a secondary carbocation. This carbocation rearranges via a hydride shift to form a more stable tertiary carbocation. Finally, the chloride ion attacks the tertiary carbocation to yield the product. The initial reactant is 1-cyclohexyl-1-methylpropan-2-ol.
- Protonation: The hydroxyl group is protonated by HCl.
- Carbocation formation: Water leaves, forming a secondary carbocation at the carbon bearing the -CH(CH₃) group.
- Rearrangement: A hydride shift from the adjacent carbon (bearing the -CH₃ group) to the carbocation center forms a tertiary carbocation at the carbon that was originally bonded to the hydroxyl group.
- Nucleophilic attack: The chloride ion attacks the tertiary carbocation.
The final product is 2-chloro-2-(cyclohexyl)propane, which corresponds to option (1).
