Question
Question: OH NH, Cl NH 2 NH Cl [4,-1] (a) Find out the total number of 1° hydrgoen. (b) Find out the total nu...
OH
NH, Cl NH 2 NH Cl [4,-1] (a) Find out the total number of 1° hydrgoen. (b) Find out the total number of 2º hydrogen (c) Find out the total number of 3° hydrogen (d) Find out the total number of 1° carbon. (e) Find out the total number of 2° carbon. (f) Find out the total number of 3° carbon. (g) Find out the total number of 4º carbon. (h) Find out the total number of type functional groups.
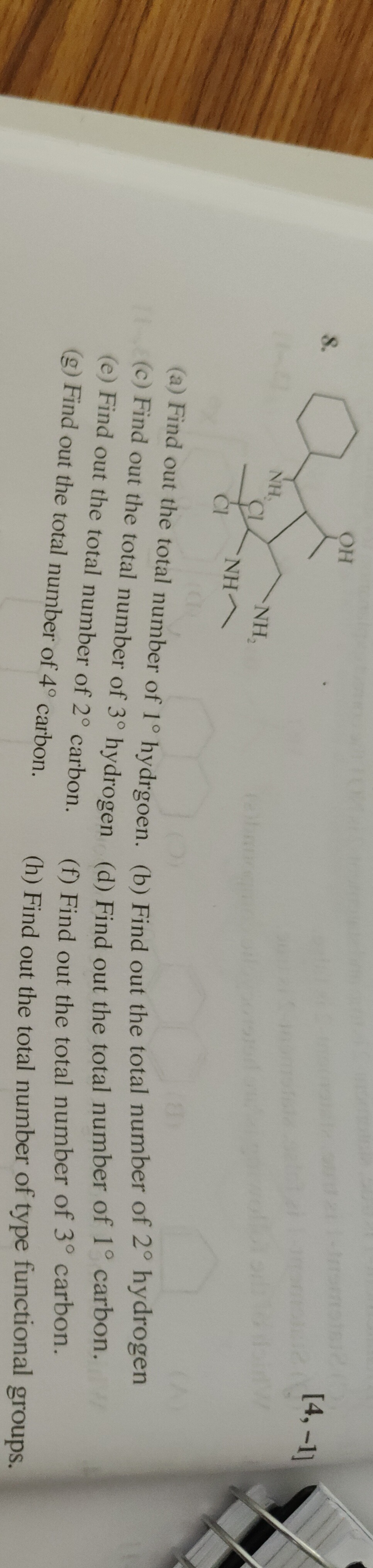
(a) Total number of 1° hydrogen: 3 (b) Total number of 2° hydrogen: 14 (c) Total number of 3° hydrogen: 1 (d) Total number of 1° carbon: 2 (e) Total number of 2° carbon: 8 (f) Total number of 3° carbon: 1 (g) Total number of 4° carbon: 0 (h) Total number of type functional groups: 4
Solution
To solve this problem, we need to first draw the complete structural formula of the given compound, explicitly showing all carbon and hydrogen atoms. Then, we will classify each carbon atom as primary (1°), secondary (2°), tertiary (3°), or quaternary (4°) based on the number of other carbon atoms it is directly bonded to. Subsequently, we will classify hydrogen atoms based on the type of carbon they are attached to. Finally, we will identify all distinct types of functional groups present in the molecule.
1. Drawing the complete structure and numbering carbons:
Let's represent the structure clearly. The given structure is a complex one involving a cyclohexane ring and a branched chain with various functional groups.
Let's write down the carbons and their attached hydrogens and their connections for classification:
- C1 (Cyclohexane): Bonded to C2, C6, C7. This carbon forms 3 C-C bonds. It also has 1 hydrogen atom (to complete its valency of 4).
- C2 (Cyclohexane): Bonded to C1, C3. This carbon forms 2 C-C bonds. It has 2 hydrogen atoms.
- C3 (Cyclohexane): Bonded to C2, C4. This carbon forms 2 C-C bonds. It has 2 hydrogen atoms.
- C4 (Cyclohexane): Bonded to C3, C5. This carbon forms 2 C-C bonds. It has 2 hydrogen atoms.
- C5 (Cyclohexane): Bonded to C4, C6. This carbon forms 2 C-C bonds. It has 2 hydrogen atoms.
- C6 (Cyclohexane): Bonded to C5, C1. This carbon forms 2 C-C bonds. It has 2 hydrogen atoms.
- C7 (Chain): Bonded to C1, C8. This carbon forms 2 C-C bonds. It has 1 hydrogen atom (and an -OH group).
- C8 (Chain): Bonded to C7, C9. This carbon forms 2 C-C bonds. It has 1 hydrogen atom (and an -NH2 group).
- C9 (Chain): Bonded to C8, C10. This carbon forms 2 C-C bonds. It has 2 hydrogen atoms.
- C10 (Chain): Bonded to C9. This carbon forms 1 C-C bond. It has 0 hydrogen atoms (and two -Cl groups and an -NHCH3 group).
- C11 (Methyl group): Bonded to the nitrogen atom of the -NHCH3 group. It is not directly bonded to any carbon atom. However, a methyl carbon (CH3) is conventionally classified as a primary (1°) carbon as it represents the end of an alkyl chain. It has 3 hydrogen atoms.
2. Classification of Carbons and Hydrogens:
-
1° Carbon: A carbon atom bonded to only one other carbon atom.
- C10 (bonded to C9) - 1 carbon
- C11 (methyl carbon, conventionally 1°) - 1 carbon
- Total 1° carbons = 2
-
2° Carbon: A carbon atom bonded to two other carbon atoms.
- C2, C3, C4, C5, C6 (5 carbons from the cyclohexane ring)
- C7, C8, C9 (3 carbons from the chain)
- Total 2° carbons = 5 + 3 = 8
-
3° Carbon: A carbon atom bonded to three other carbon atoms.
- C1 (bonded to C2, C6, C7) - 1 carbon
- Total 3° carbons = 1
-
4° Carbon: A carbon atom bonded to four other carbon atoms.
- None
- Total 4° carbons = 0
-
1° Hydrogen: Hydrogen atoms attached to 1° carbons.
- From C11 (CH3): 3 hydrogens
- Total 1° hydrogens = 3
-
2° Hydrogen: Hydrogen atoms attached to 2° carbons.
- From C2, C3, C4, C5, C6 (5 carbons * 2 H/carbon) = 10 hydrogens
- From C7 (CH): 1 hydrogen
- From C8 (CH): 1 hydrogen
- From C9 (CH2): 2 hydrogens
- Total 2° hydrogens = 10 + 1 + 1 + 2 = 14
-
3° Hydrogen: Hydrogen atoms attached to 3° carbons.
- From C1 (CH): 1 hydrogen
- Total 3° hydrogens = 1
3. Functional Groups:
Identify distinct types of functional groups present in the molecule.
- -OH: Hydroxyl group (Alcohol)
- -NH2: Primary amine (Nitrogen bonded to one carbon and two hydrogens)
- -Cl: Alkyl halide (Chlorine atom bonded to a carbon). Although there are two -Cl atoms, they represent one type of functional group.
- -NHCH3: Secondary amine (Nitrogen bonded to two carbons, C10 and C11, and one hydrogen).
Total number of types of functional groups = 4.
Summary of Answers:
(a) Total number of 1° hydrogen: 3 (b) Total number of 2° hydrogen: 14 (c) Total number of 3° hydrogen: 1 (d) Total number of 1° carbon: 2 (e) Total number of 2° carbon: 8 (f) Total number of 3° carbon: 1 (g) Total number of 4° carbon: 0 (h) Total number of type functional groups: 4
The final answer is a)3,b)14,c)1,d)2,e)8,f)1,g)0,h)4
Explanation of the solution:
- Draw the complete structure: Implicit hydrogens are added to each carbon to satisfy its valency of four.
- Classify Carbons:
- 1° carbon: bonded to one other carbon. (C10, C11)
- 2° carbon: bonded to two other carbons. (C2, C3, C4, C5, C6, C7, C8, C9)
- 3° carbon: bonded to three other carbons. (C1)
- 4° carbon: bonded to four other carbons. (None)
- Count Hydrogens:
- 1° hydrogen: attached to 1° carbon. (3 H on C11)
- 2° hydrogen: attached to 2° carbon. (10 H from C2-C6, 1 H from C7, 1 H from C8, 2 H from C9 = 14 H)
- 3° hydrogen: attached to 3° carbon. (1 H on C1)
- Identify Functional Groups: List all distinct functional groups present. (Alcohol, Primary Amine, Alkyl Halide, Secondary Amine).
Answer: (a) Total number of 1° hydrogen: 3 (b) Total number of 2° hydrogen: 14 (c) Total number of 3° hydrogen: 1 (d) Total number of 1° carbon: 2 (e) Total number of 2° carbon: 8 (f) Total number of 3° carbon: 1 (g) Total number of 4° carbon: 0 (h) Total number of type functional groups: 4
