Question
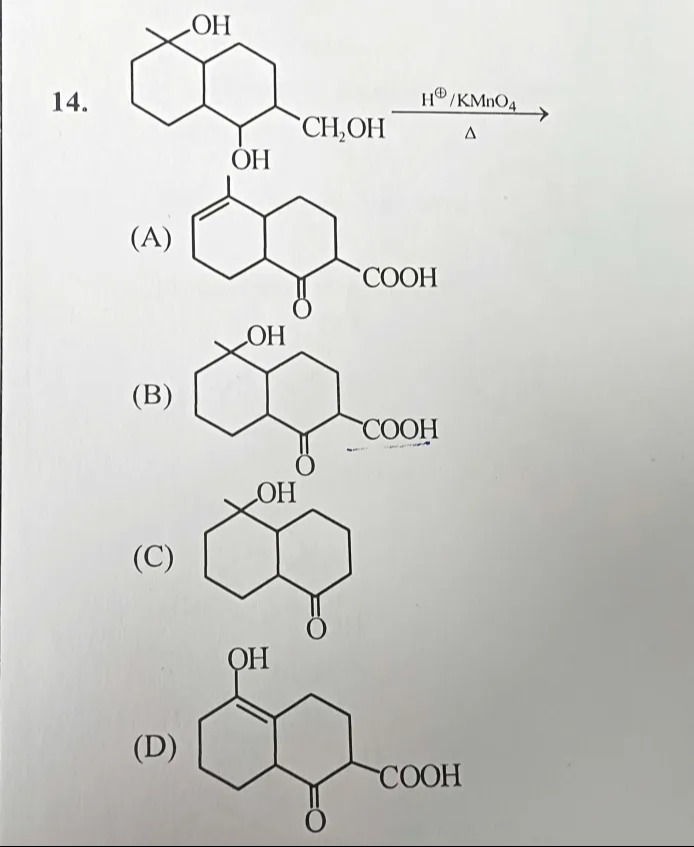
Question: OH 14. <figure description="The figure shows a chemical reaction scheme. The reactant is a bicycl...
OH
H⊕/KMnO₄ Δ
A
COOH
OH
B
COOH
OH
C
D
COOH
Answer
(B)
Explanation
Solution
The starting bicyclic compound has two parts:
-
One six‐membered ring with a carbon bearing both a methyl group and a hydroxyl group. Since this carbon (likely tertiary) lacks an oxidizable hydrogen, it remains unchanged under oxidation.
-
The other six‐membered ring has a vicinal diol: a secondary hydroxyl group and a primary –CH₂OH. Under the vigorous conditions (H⁺/KMnO₄, Δ), the primary –CH₂OH is oxidized to –COOH and the secondary –OH is oxidized to a ketone.
Thus, the oxidation gives a bicyclic system (two fused six‐membered rings) where one ring retains the methyl and –OH while the other ring now carries a ketone and a carboxylic acid group—this corresponds to product (B).
