Question
Question: Of the following, which is the product formed when cyclohexanone undergoes aldol condensation follow...
Of the following, which is the product formed when cyclohexanone undergoes aldol condensation followed by heating?
A. 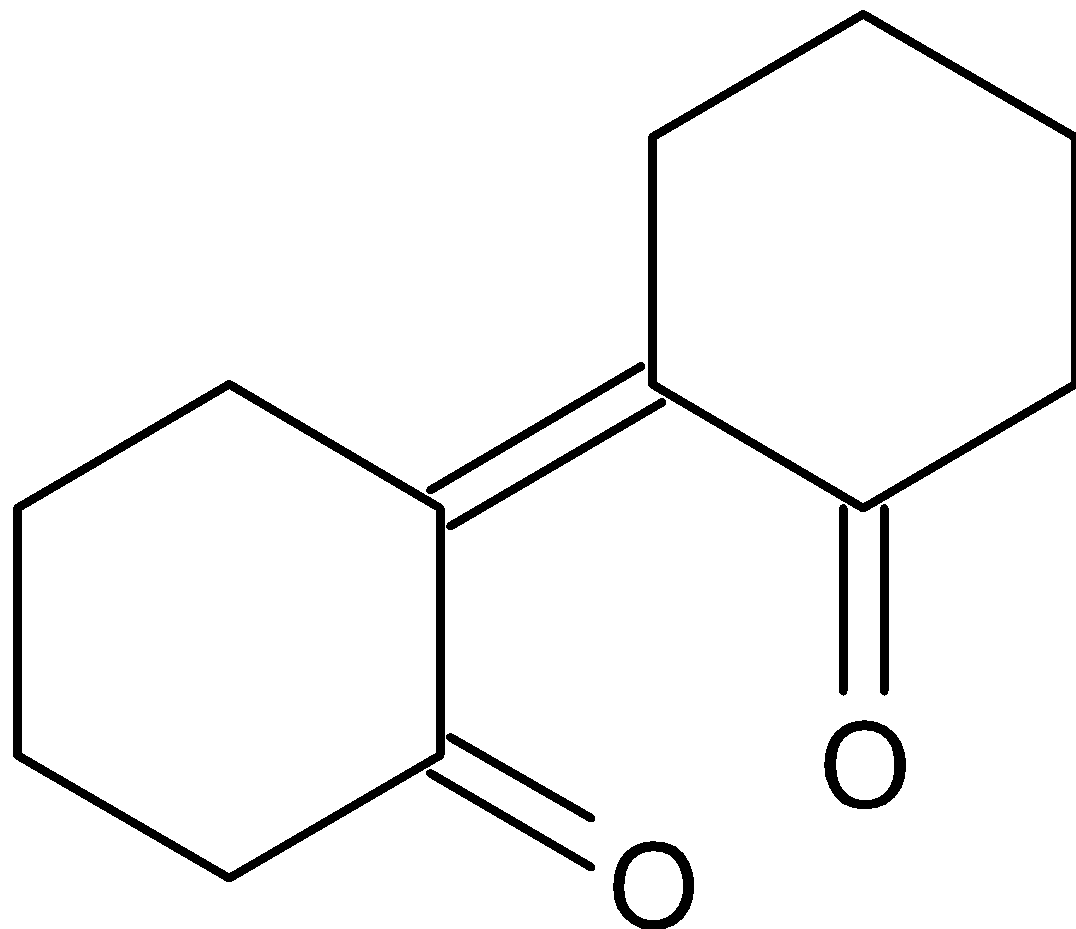
B. 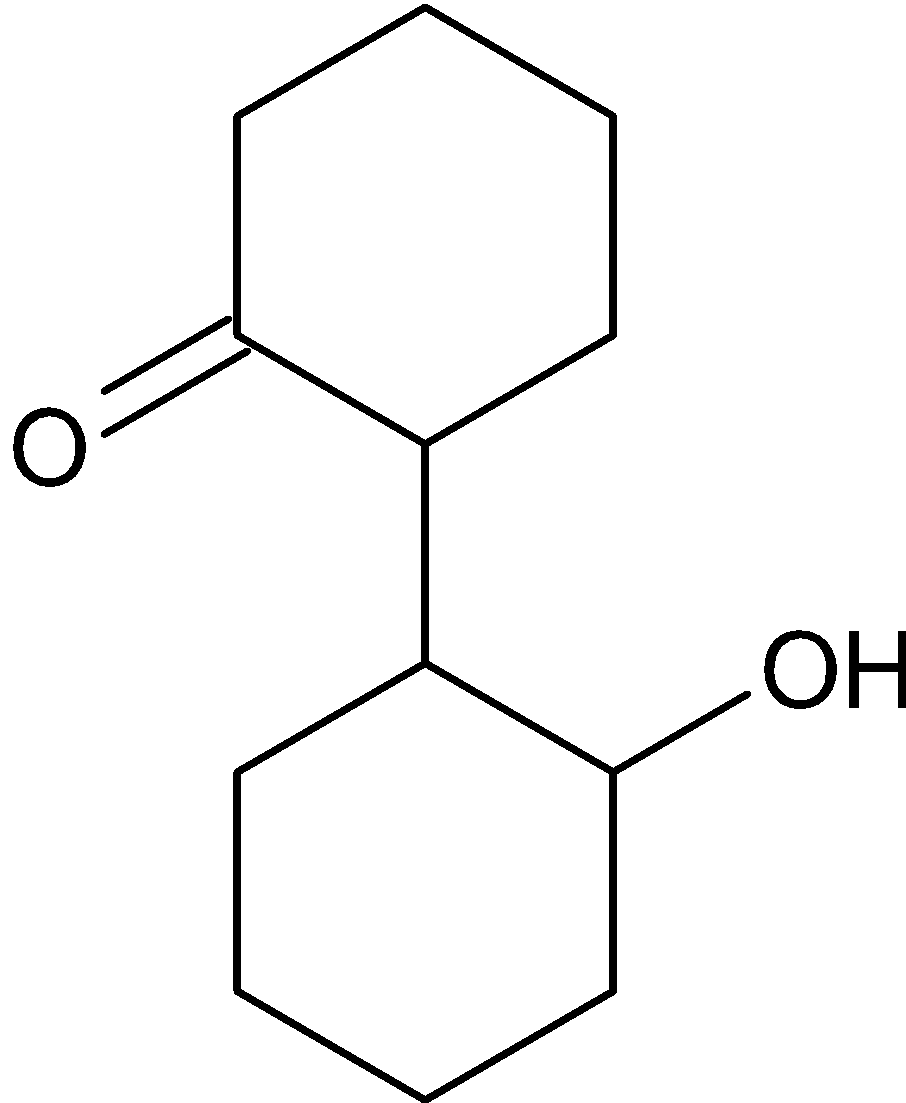
C. 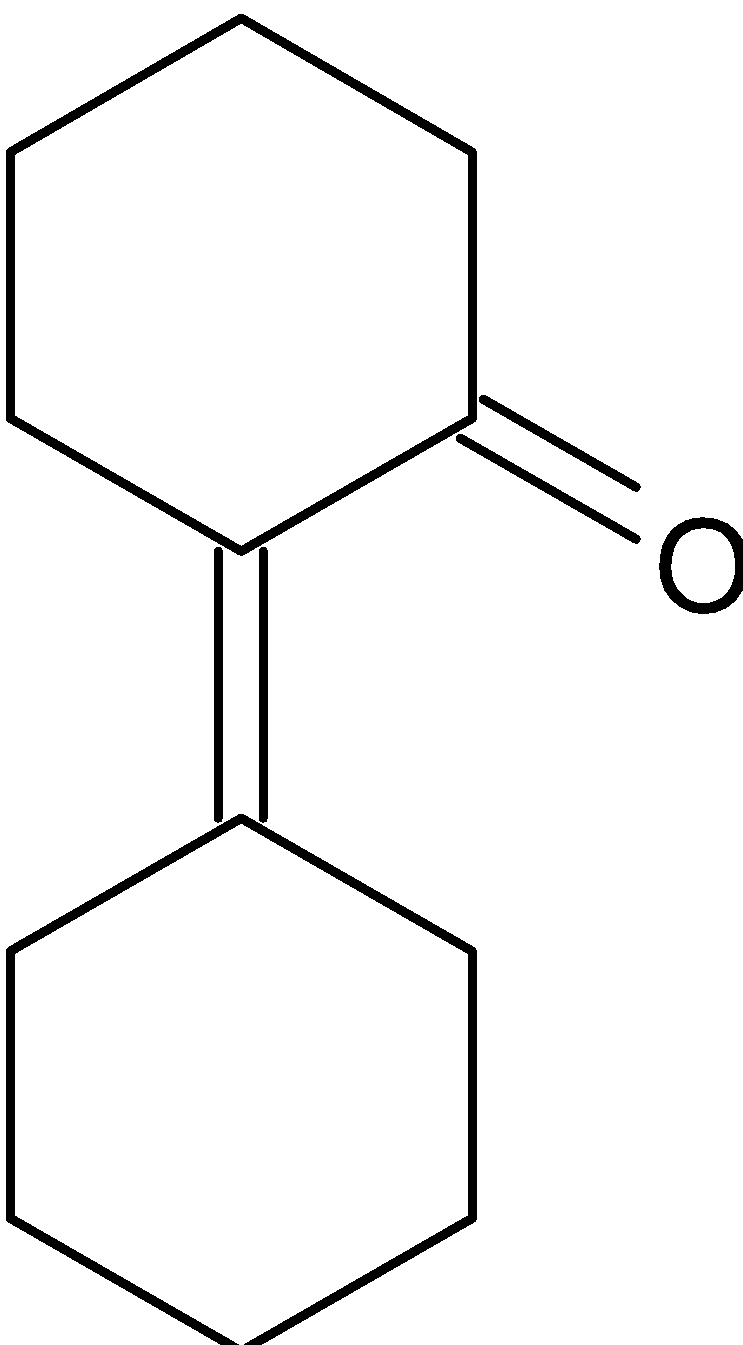
D. 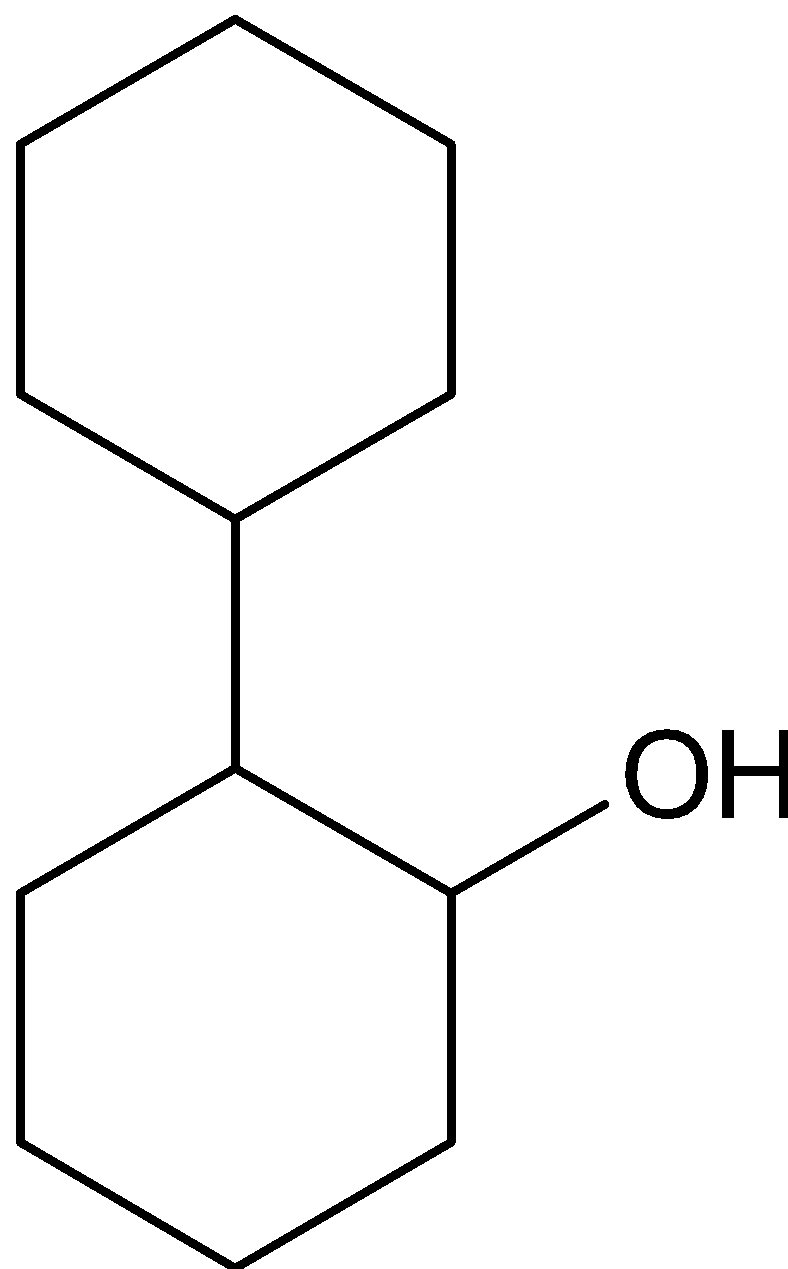
Solution
Condensation between two molecules of an aldehyde or ketone to form a β− hydroxy aldehyde or a β− hydroxy ketone is called aldol condensation. It is possible only when the carbonyl compound contains at least one α− hydrogen atom.
Complete step by step answer:
Aldol condensation is a property of carbonyl compounds having hydrogen at α− carbon atom. Aldol means aldehyde and alcohol groups on the same molecule. It may occur between two aldehydes or ketones in the presence of a catalytic base. The reaction is only possible between two components having α− hydrogen.
When cyclohexanone undergoes aldol condensation in the presence of a base, it will produce a β− hydroxy ketone.
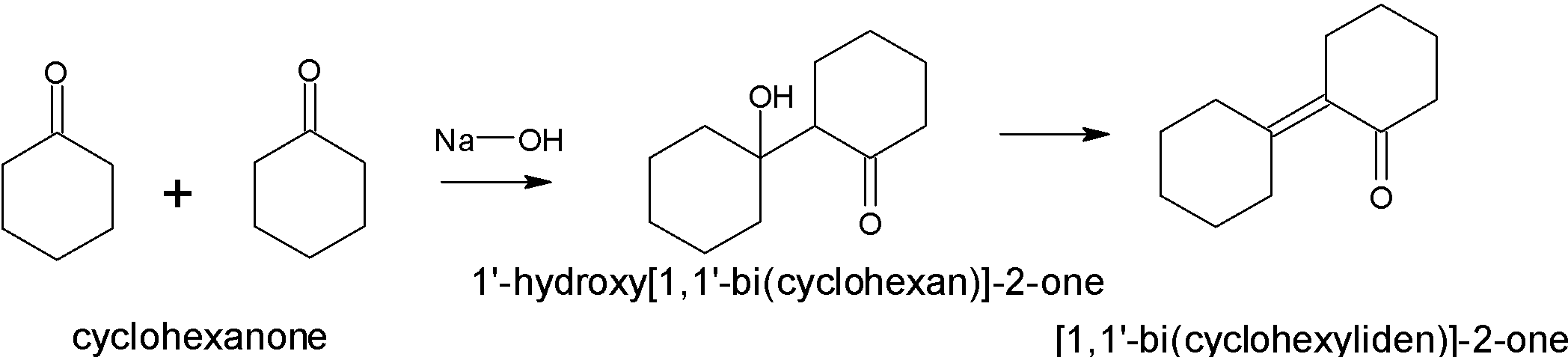
The mechanism involves three steps:
-First is the acid-base reaction. Hydroxide functions as a base and removes the acidic α− hydrogen giving the reactive enolate.
-The nucleophilic enolate anion attacks the ketone at the electrophilic carbonyl carbon in a nucleophilic addition giving an intermediate alkoxide. Alkoxide ion is the conjugate base of alcohols.
-The alkoxide deprotonates a water molecule creating hydroxide and the aldol is formed.
-Addition of water results in the formation of condensation products.
-Water molecules are removed by heating in the presence of acid. This makes a double bond.
Thus the product formed is a α,β− unsaturated alkene.
Hence the correct option is C.
Note:
Some conditions regarding the aldol condensation are:
A reversible equilibrium
OH is the base typically used in an aldol reaction.
Can be carried out either by aldehydes or ketones.
With aldehydes, the equilibrium favors products.
With ketones, the equilibrium favors the reactants.
