Question
Question: Of the following molecules the ones which are hypervalent in their dimeric form: A. \( Cl{O_3},Ga{...
Of the following molecules the ones which are hypervalent in their dimeric form:
A. ClO3,GaH3,ICl3
B. ClO3,ICl3,Co(CO)4
C. OF,NO2,GaH3
D. GaH3,ClO3,ICl3,Co(CO)4
Solution
Hint : Dimerization is the process when two molecules of similar chemical composition come together to form a single polymer called a dimer. Oligomers containing two monomers which are joined by bonds, which are either strong, weak or covalent or intermolecular are called dimers while dimers containing two identical molecules are called homodimer whereas dimers which do not contain identical molecules are called heterodimer.
Complete Step By Step Answer:
Hypervalent are molecules which contain elements having more than eight electrons in its valence shell whereas, hypovalent are molecules which contain elements having less than eight electrons in its valence shell.
ClO3 ClO3 is hypervalent in its dimeric form because in this compound the central atom i.e. Chlorine has more than eight in its valence shell hence, this molecule is hypervalent.
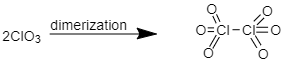
ICl3 is also a hypervalent in its dimeric form because the central atom i.e. Iodine has more than eight electrons in its valence shell hence, this molecule is hypervalent.
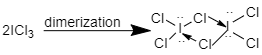
Co(CO)4 is hypervalent in dimeric form because the central atom i.e. Cobalt has more than eight electrons in its valence shell, therefore this molecule is also hypervalent.
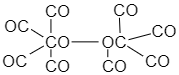
GaH3 is hypovalent in its dimeric form because the central atom i.e. Gallium has less than eight electrons in its valence shell hence, this compound is hypovalent.
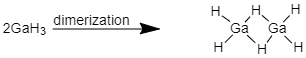
When 2OF undergoes dimerization the molecule has complete octet in dimer form.
Therefore the correct answer is option B.
Note :
There is a characteristic difference in the structure of trihalides of boron and aluminium. AlCl3 exists as dimer Al2Cl6 while BeCl3 exists as monomer, both B and Al are electron deficient compounds and behave as Lewis acid but Aluminium chloride exists as dimer whereas boron chloride exists as monomer.
