Question
Question: Of the following molecules, the ones which are hypervalent in their dimeric form: \( Cl{O_3}\,,\,...
Of the following molecules, the ones which are hypervalent in their dimeric form:
ClO3,OF,GaH3,AlCl3,ICl3,NO2,Co(CO)4
(A) ClO3,GaH3,ICl3
(B) ClO3,ICl3,Co(CO)4
(C) OF,NO2,GaH3
(D) GaH3,ClO3,ICl3,Co(CO)4
Solution
Hint : Hypervalent, the name itself indicates that there are more electrons in the valence shell of the molecules. This phenomenon is also termed as expanded octet. A dimer is formed when two monomers are joined by a bond.
Complete Step By Step Answer:
We know that the outermost electron shell of an atom which contains valence electrons is termed as valence shell. These electrons participate in the chemical bond formation. These valence electrons help in determining the chemical properties of an element. In the case of the main group element, valence electrons exist only in the outermost electron shell whereas in case of transition metal, a valence electron can also exist in the inner shell.
A hypervalent molecule which contains one or more main group elements that contain more than eight electrons in the valence shell of the main group elements. There are some specific classes of hypervalent molecules such as noble gas compounds like XeF4 , tetra-, penta- and hexavalent phosphorous, silicon and sulfur compounds like PCl5,SF6 and hypervalent iodine like IF7 etc.
Now, we have to predict the hypervalent molecules in dimeric form. So, first we should know about the dimeric form. A dimeric form is an oligomer which is formed by joining two monomers by a bond. This bond may be covalent or intermolecular, strong or weak. When the two monomers are same then it is known as homodimer whereas when the two monomers are different then, it is known as heterodimer.
Make structures of the dimers of the molecules to know whether it is hypervalent or not. In the given question, there are only three dimers of molecules which exist as hypervalent..
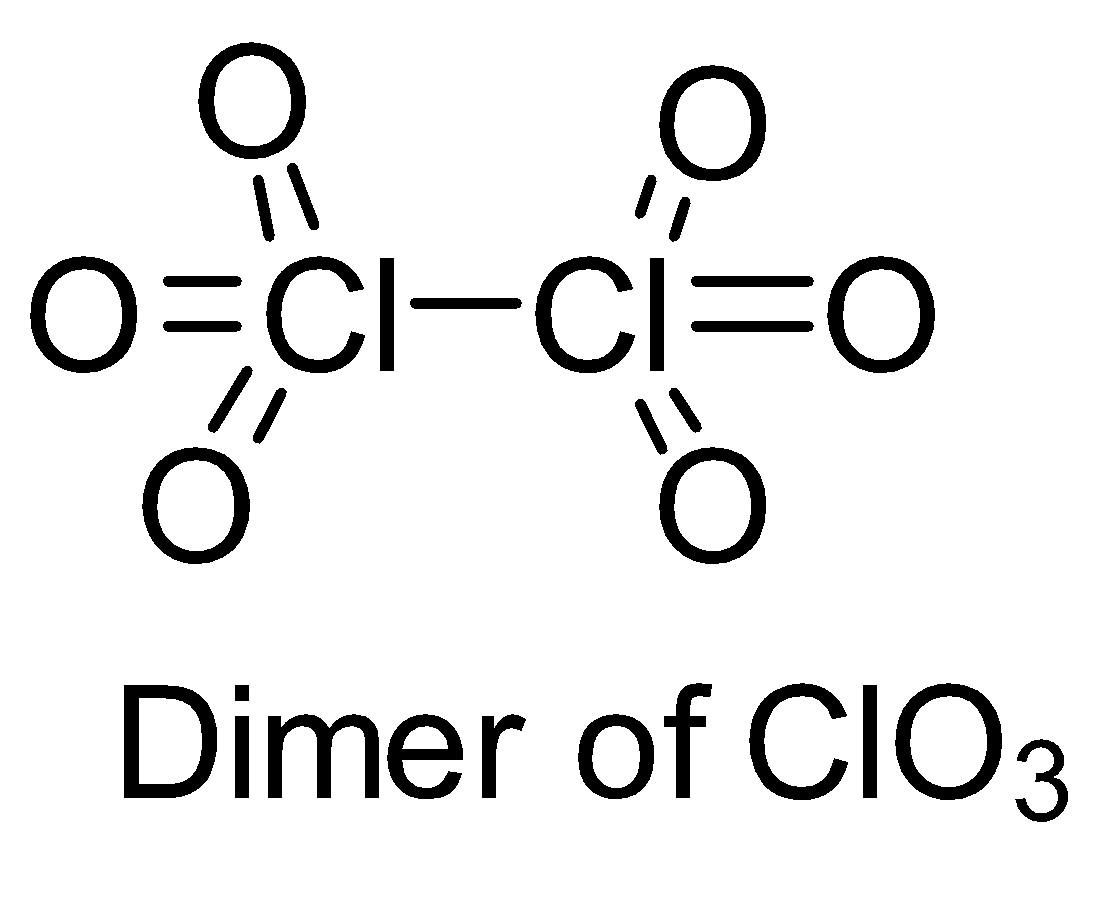
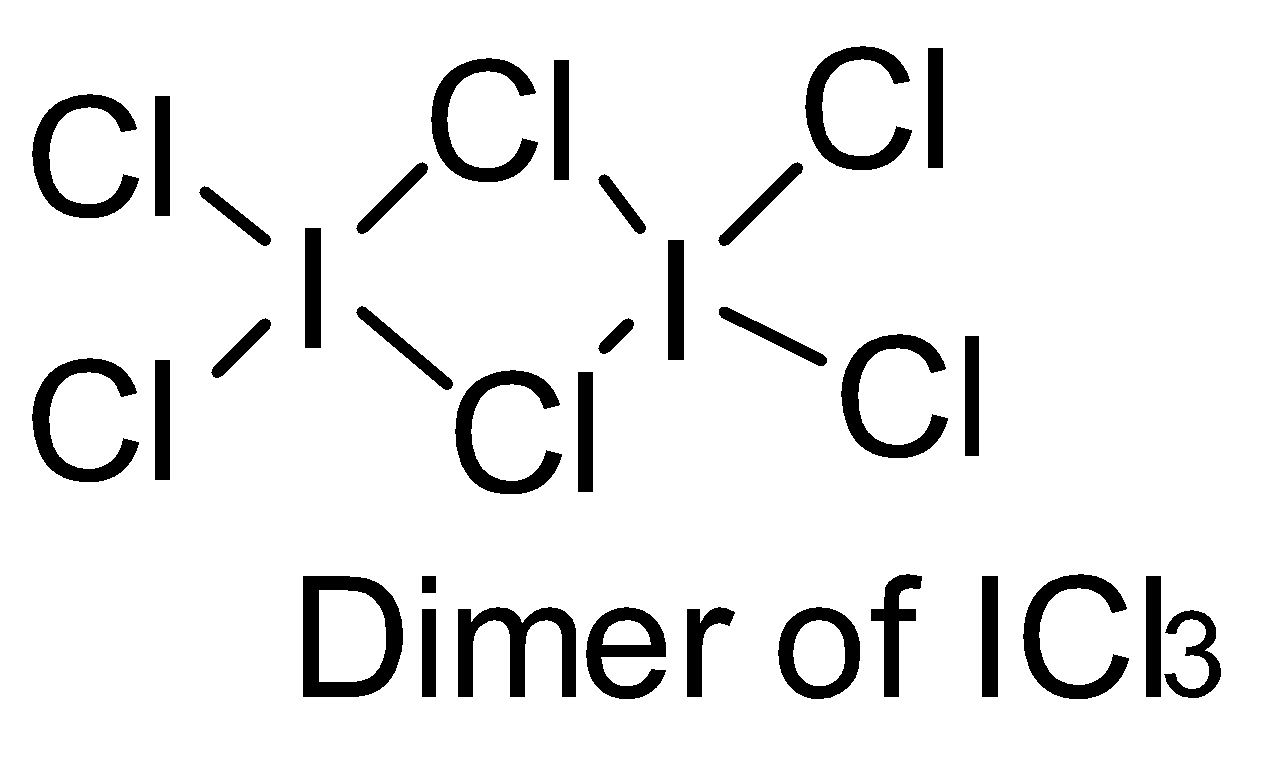
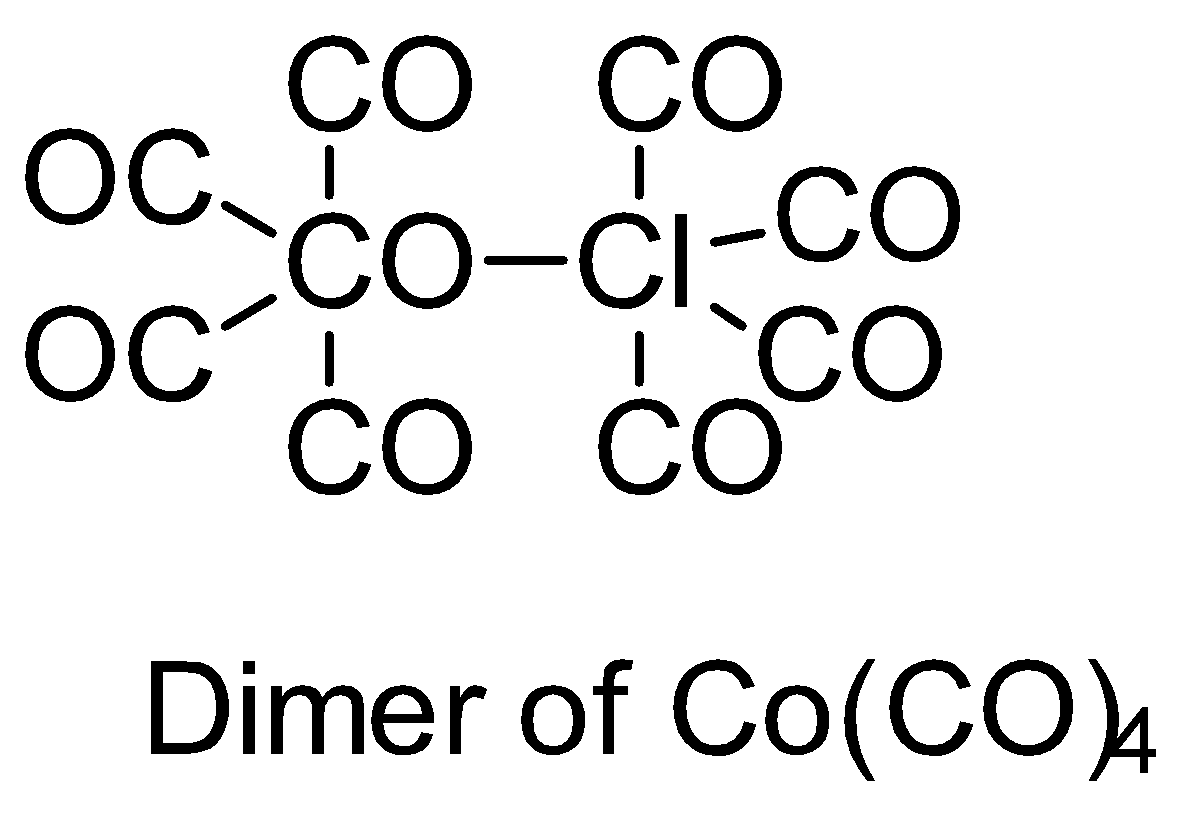
Hence, the correct answer is option (B).
Note :
Hypervalency is a concept based on hybrid orbital theory and Lewis theory. It is useful in predicting the molecular shape and how atoms are connected to each other. Dimerization occurs when two molecules dimerise by a bond and the reverse of dimerization is called dissociation.
