Question
Question: Of the 3 states of matter, solid, liquid and gas, which has the lowest kinetic energy for a given su...
Of the 3 states of matter, solid, liquid and gas, which has the lowest kinetic energy for a given substance?
Solution
We know that the kinetic energy is the energy that an object possesses due to its random motion and the particles of matter are in continuous motion and thus generate kinetic energy by moving continuously. The temperature of any substance is a measure of the average kinetic energy of the molecules.
Complete answer:
To predict order of kinetic energy in three states of matter, we first need to give main points of difference between these three states i.e., solid, liquid and gas which is as follows:
| Solid | Liquid | Gas |
|---|---|---|
| Molecules are held very close to each other forming a close packed structure. The molecule tends to bend or vibrate but remains in a close proximity and has an ordered arrangement. | Molecules have the tendency to flow over one another but stay towards the bottom of the container. Motion of the molecules is a bit more random as compared to solid. The kinetic energy of these particles is enough to slip over the ordered arrangement of a solid. | Molecules are in continuous straight-line motion. The kinetic energy of the molecules is greater than the attractive forces between the particles thus, the particles are very far from each other and tend to show random motion. |
 | 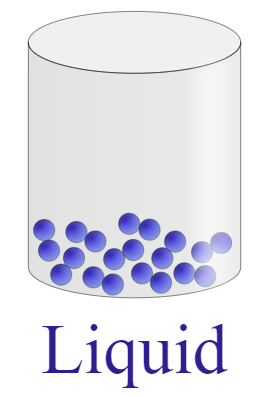 | 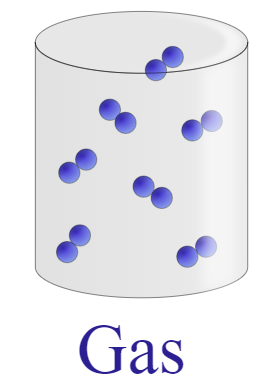 |
It is clear from the difference table that the particles of solid show least random motion while particles of gas have highest degree of random motion and we know that kinetic energy is the energy of an object performing random motion that means higher the motion of particles, greater will be the energy.
So, in reference to this we can say that the solids will have the least amount of kinetic energy because the particles inside a solid are very tightly packed due to strong intermolecular forces existing between the particles and due to this, the particles are not available for doing random motion. On the other hand, gas particles have large space between them and they experience less intermolecular forces and move with comparatively higher velocity. Hence, gases have the highest amount of kinetic energy.
Thus, we can conclude that solid has the lowest kinetic energy for a given substance.
Note:
Remember that the kinetic energy of the molecules also depends on the temperature as on increasing temperature, the particles tend to move faster and the velocity of random motion increases, due to which the kinetic energy of the particle will be increased as well. So, generally an increase in temperature will increase the average kinetic energy of the particles.
