Question
Question: Of \[{\text{P}}{{\text{H}}_3}\] and \[{{\text{H}}_{\text{2}}}{\text{S}}\] which is more acidic and w...
Of PH3 and H2S which is more acidic and why?
Solution
The acidity of a molecule depends upon the polarity of the bond. More polar bonds cause the more acidity of the molecule. Electronegativity is defined as the power of an atom to attract the bonded electrons toward itself.
Complete answer:
Acidity is defined as the strength of acid to donate a proton. More acidity means more is the power to release protons.
If an atom has more electronegative it will attract the electrons towards itself, so it will get a negative charge and another bonded atom will get a positive charge. So, on increasing electronegativity difference the polarity of bond increases.
If another atom is hydrogen then the hydrogen will release in the form of a proton when the bond will break.
So, as the polarity increases the acidity of the acid increases.
In phosphorus hydride, the central atom is phosphorous and in hydrogen sulphide, the central atom is sulphur.
The structure of the phosphorus hydride and hydrogen sulphide is as follows:
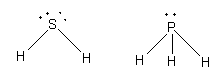
The electronegative of phosphorus is 2.2 and sulphur is 2.6. So, the electronegativity of sulphur is more than phosphorus and thus sulphur-hydrogen bond is more polar than phosphorus-hydrogen bond.
So, the acidity of hydrogen sulphide is more than the acidity of phosphorus hydride.
Therefore, H2S is more acidic due to a more polar sulphur-hydrogen bond.
Note: In a period of p-block, the electronegativity increases from right to left thus, the polarity of non-metal and hydrogen bonds increases, so the acidity of non-metal hydride increases from right to left in a period.
