Question
Question: Find the number of reaction(s) in which coordinate bond is/are formed in product side:...
Find the number of reaction(s) in which coordinate bond is/are formed in product side:
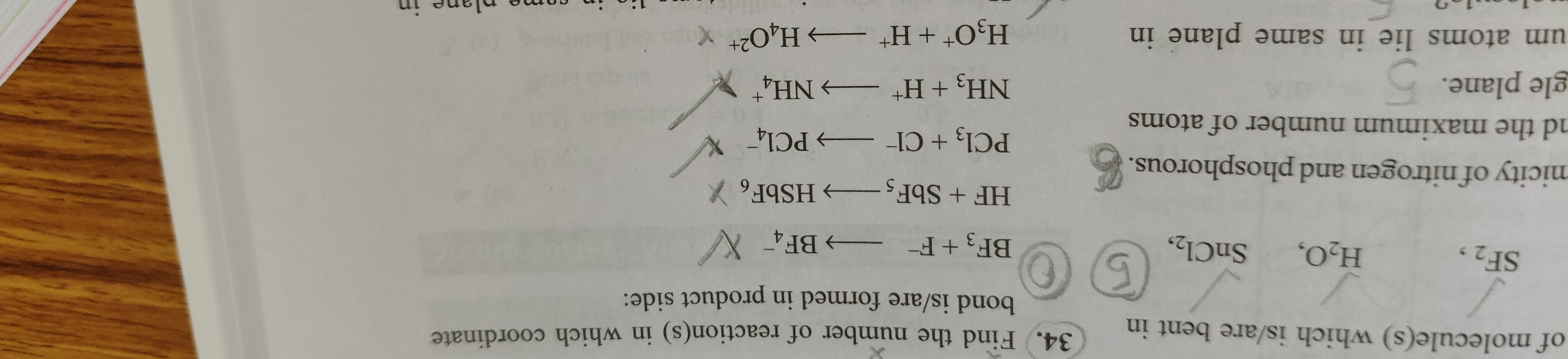
5
Solution
A coordinate bond (or dative bond) is a type of covalent bond where both shared electrons are contributed by only one of the participating atoms. This typically occurs in Lewis acid-base reactions where a Lewis base (electron pair donor) reacts with a Lewis acid (electron pair acceptor).
-
BF3+F−⟶BF4−:
BF3 is a Lewis acid, and F− is a Lewis base. F− donates a lone pair to the empty p-orbital of Boron in BF3 to form a B-F bond. Coordinate bond is formed. -
HF+SbF5⟶HSbF6:
SbF5 is a strong Lewis acid. HF acts as a source of F−. The F− ion coordinates to SbF5 to form the stable [SbF6]− anion. The bond between Sb and the incoming F is a coordinate bond. Coordinate bond is formed. -
PCl3+Cl−⟶PCl4−:
PCl3 has a lone pair on Phosphorus, and Phosphorus has available empty d-orbitals. Cl− is a Lewis base. Cl− donates a lone pair to Phosphorus, forming a P-Cl bond and expanding Phosphorus's octet. Coordinate bond is formed. -
NH3+H+⟶NH4+:
NH3 is a Lewis base because Nitrogen has a lone pair of electrons. H+ is a Lewis acid. The lone pair on Nitrogen in NH3 is donated to the empty 1s orbital of H+ to form a N-H bond. Coordinate bond is formed. -
H3O++H+⟶H4O22+:
H3O+ has one lone pair remaining on the Oxygen atom. H+ is a Lewis acid. The remaining lone pair on Oxygen in H3O+ is donated to the incoming H+. Coordinate bond is formed.
All five reactions involve the formation of a coordinate bond in the product side.
