Question
Question: Observe the following two set/s of reactions (I and II). Select the true and false statements about ...
Observe the following two set/s of reactions (I and II). Select the true and false statements about the ratio of their rate constants (K).
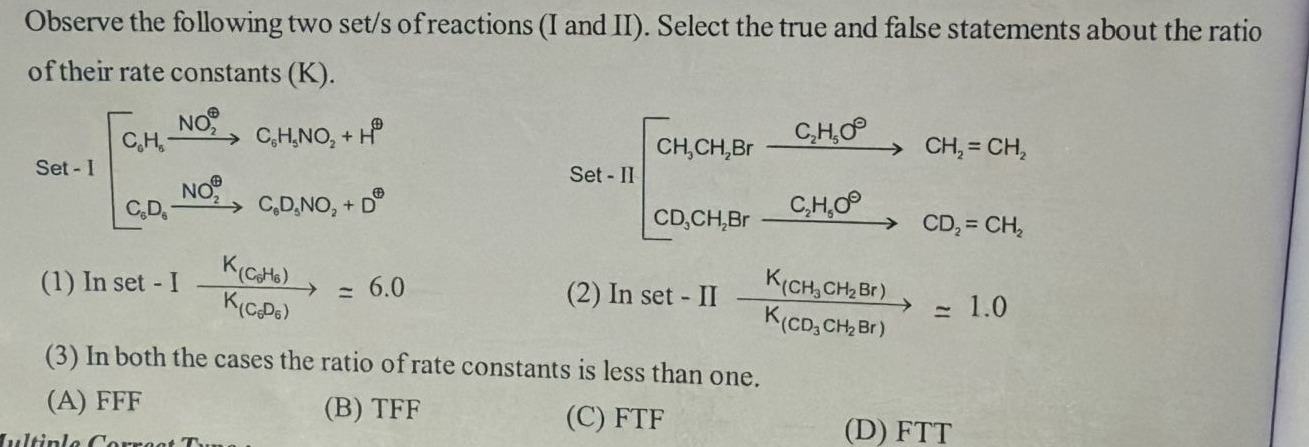
In set - I K(C6D6)K(C6H6) = 6.0 (2) In set - II K(CD3CH2Br)K(CH3CH2Br) ≃ 1.0
(3) In both the cases the ratio of rate constants is less than one. (A) FFF
In set - I K(C6D6)K(C6H6) = 6.0 (2) In set - II K(CD3CH2Br)K(CH3CH2Br) ≃ 1.0
(3) In both the cases the ratio of rate constants is less than one. (B) TFF
In set - I K(C6D6)K(C6H6) = 6.0 (2) In set - II K(CD3CH2Br)K(CH3CH2Br) ≃ 1.0
(3) In both the cases the ratio of rate constants is less than one. (C) FTF
In set - I K(C6D6)K(C6H6) = 6.0 (2) In set - II K(CD3CH2Br)K(CH3CH2Br) ≃ 1.0
(3) In both the cases the ratio of rate constants is less than one. (D) FTT
TFF
Solution
The problem asks us to evaluate the truthfulness of three statements regarding the ratio of rate constants (K) for two sets of reactions involving isotopic substitution. This phenomenon is related to the kinetic isotope effect (KIE).
Set I: Electrophilic nitration of benzene and hexadeuterobenzene.
C6H6+NO2+→C6H5NO2+H+
C6D6+NO2+→C6D5NO2+D+
This is an electrophilic aromatic substitution reaction. The generally accepted mechanism involves the formation of a sigma complex in the rate-determining step, followed by rapid deprotonation to restore aromaticity.
C6H6+NO2+slow[sigmacomplex]fastC6H5NO2+H+
C6D6+NO2+slow[deuteratedsigmacomplex]fastC6D5NO2+D+
If the formation of the sigma complex is the rate-determining step, the C-H/C-D bond breaking occurs in a fast step, and the primary kinetic isotope effect is expected to be small, close to 1. However, if the deprotonation step is partially or fully rate-determining, a primary kinetic isotope effect will be observed, where the reaction with C-H is faster than with C-D. The magnitude of the primary KIE (K(C-H)/K(C-D)) is typically in the range of 2-8 at room temperature. Statement (1) claims that in Set I, K(C6D6)K(C6H6)=6.0. A value of 6.0 is a significant primary kinetic isotope effect, suggesting that the C-H/C-D bond breaking is involved in the rate-determining step. While for nitration of benzene, the KIE is usually small, the given value indicates a scenario where deprotonation is at least partially rate-determining. Assuming the given value is correct, statement (1) is True.
Set II: Elimination reaction of ethyl bromide and trideuteroethyl bromide with ethoxide.
CH3CH2Br+C2H5O−→CH2=CH2+C2H5OH+Br−
CD3CH2Br+C2H5O−→CD2=CH2+C2H5OH+Br−
These are likely E2 elimination reactions. In an E2 reaction, a base abstracts a β-hydrogen (or deuterium), the C-H/C-D bond breaks, the C-Br bond breaks, and a C=C double bond forms in a concerted manner.
In the first reaction, a proton is abstracted from the CH₃ group (β-carbon) and Br is removed from the CH₂ group (α-carbon).
In the second reaction, a deuteron is abstracted from the CD₃ group (β-carbon) and Br is removed from the CH₂ group (α-carbon). The product shown CD₂=CH₂ implies that deuterium is removed from the CD₃ group and bromine from the CH₂ group.
Since a C-H bond is broken in the first reaction and a C-D bond is broken in the second reaction in the rate-determining step (concerted E2), a primary kinetic isotope effect is expected. The rate constant for the reaction with CH₃CH₂Br (breaking C-H) should be greater than the rate constant for the reaction with CD₃CH₂Br (breaking C-D). Thus, K(CD3CH2Br)K(CH3CH2Br)>1. The magnitude of the primary KIE for E2 reactions is typically in the range of 2-8.
Statement (2) claims that in Set II, K(CD3CH2Br)K(CH3CH2Br)≃1.0. A ratio of approximately 1.0 indicates little to no kinetic isotope effect, which is contrary to the expectation of a significant primary KIE for an E2 reaction where the C-H/C-D bond is broken in the rate-determining step. Therefore, statement (2) is False.
Statement (3): In both the cases the ratio of rate constants is less than one.
In Set I, the ratio K(C6D6)K(C6H6)=6.0, which is greater than one.
In Set II, the ratio K(CD3CH2Br)K(CH3CH2Br) is expected to be greater than one due to primary kinetic isotope effect. Statement (2) claims it is 1.0, which is not less than one.
Since in Set I the ratio is 6.0 (> 1), statement (3) is False.
Based on the analysis:
Statement (1) is True (assuming the given value of 6.0 is correct, indicating a primary KIE).
Statement (2) is False (a primary KIE > 1 is expected for this E2 reaction).
Statement (3) is False (the ratio is greater than one in Set I, and expected to be greater than one in Set II).
Thus, the truth values of the statements (1), (2), and (3) are T, F, F respectively.
The option corresponding to TFF is (B).
Final check:
Statement (1): KIE in nitration of benzene can be significant if deprotonation is partially rate-determining. A value of 6.0 is plausible for primary KIE. So True.
Statement (2): E2 elimination involves C-H/C-D bond breaking in the transition state, leading to a primary KIE > 1. A ratio of 1.0 is not expected. So False.
Statement (3): Both ratios are expected to be > 1 (normal KIE). So False.
The final answer is TFF.
