Question
Question: Observe the following statements and choose the correct option. S1 :Inductive effect is more powerf...
Observe the following statements and choose the correct option.
S1 :Inductive effect is more powerful than mesomeric effect.
S2 :The bond polarity order of bonds a, b, and c in the given molecule (I) is c > a > b
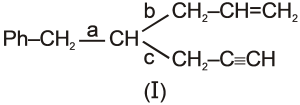
S3 :The hybrid structure has always have equal contribution from all the resonating structures.
S4 : shows inductive effect, resonance, hyperconjugation&intramolecular hydrogen bonding.
shows inductive effect, resonance, hyperconjugation&intramolecular hydrogen bonding.
A
TFTF
B
FTFT
C
FTTF
D
TTFF
Answer
FTFT
Explanation
Solution
S1 : Mesomeric effect is more dominating than inductive effect. S2 : −C≡CH>


S3 : Most stable resonating structures has maximum contribution in resonance hybrid.
S4: All effects are possible.
