Question
Question: Observe the following reaction $X \xrightarrow{NH_2OH/HCl} Y \xrightarrow{H_2SO_4} Z \xrightarrow[(...
Observe the following reaction
XNH2OH/HClYH2SO4Z(i)NaOH/Boil(ii)H+H2N−CH2−CH2−CH2−COOH
If the molar mass of X is M × 10. Then the value of M is:
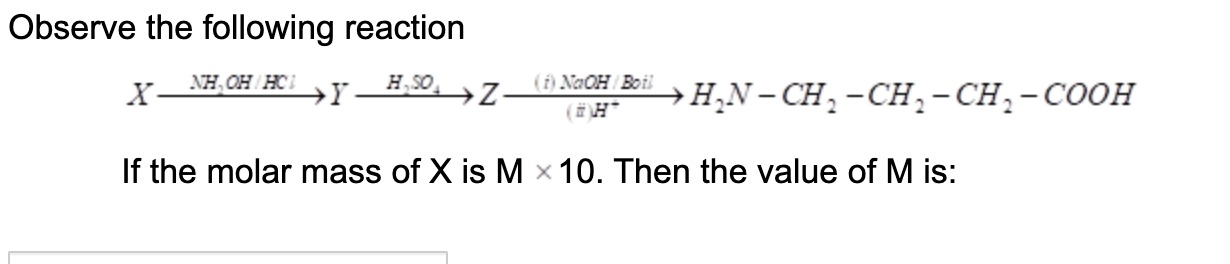
7
Solution
The given reaction sequence involves the conversion of a cyclic ketone (X) to a cyclic amide (Z) via a Beckmann rearrangement. The final product is 4-aminobutanoic acid, which is obtained by the hydrolysis of Z. This suggests that Z is a cyclic amide (lactam).
The reaction YH2SO4Z is a Beckmann rearrangement, where a cyclic ketone oxime (Y) is converted into a lactam (Z). The lactam Z is a 5-membered ring, which means it is formed from a 4-membered cyclic ketone oxime. Therefore, X is cyclobutanone (C4H6O).
The molar mass of cyclobutanone is calculated as follows: 4×12+6×1+1×16=48+6+16=70 g/mol
Given that the molar mass of X is M×10, we have: 70=M×10 M=70/10=7
Therefore, the value of M is 7.
