Question
Question: Observe the following reaction, Here, is converted to
is converted to 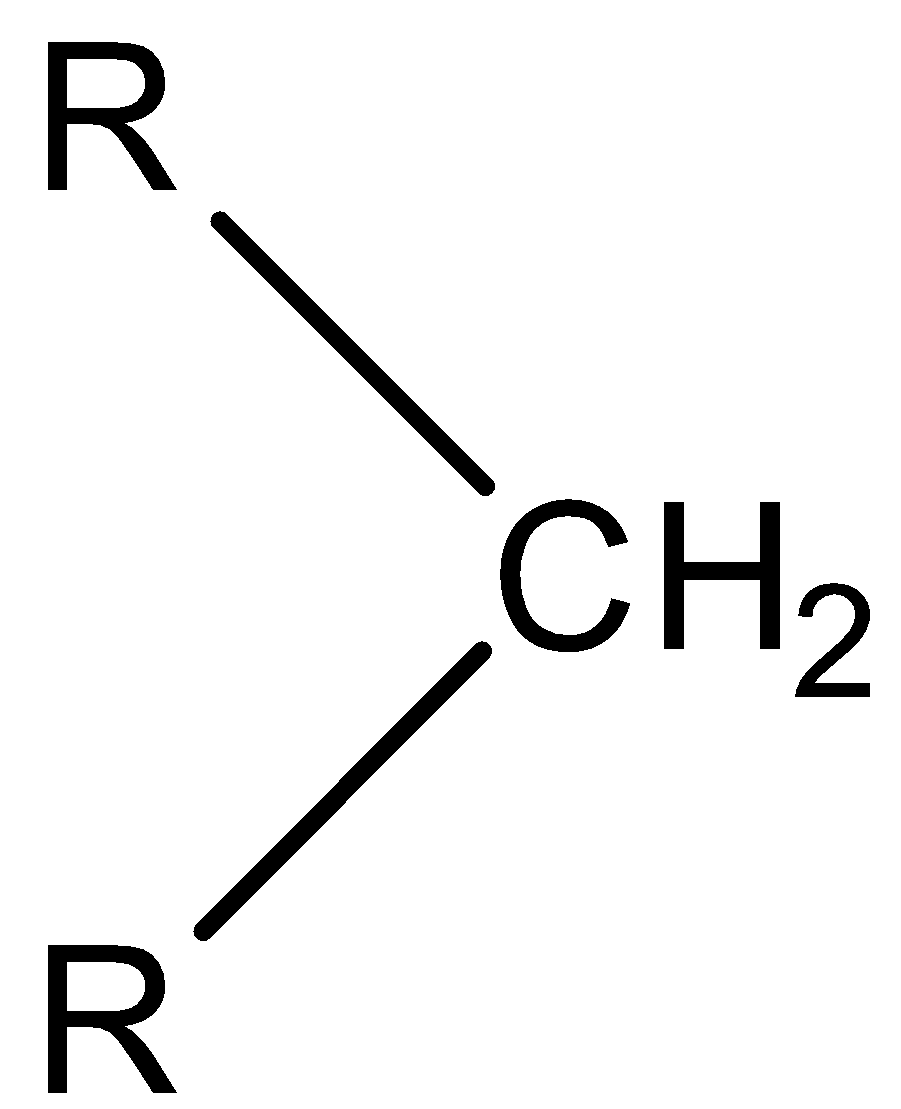 by:
by:
(a)- Wolff Kishner reduction
(b)- Clemmensen reduction
(c)- Red P + HI at 200∘C
(d)- All of these
Solution
The given reactant on the question is ketone which is a carbonyl compound and it is converted into an alkane. In Wolff-Kishner reduction, the compounds used are hydrazine, potassium hydroxide, and ethylene glycol. In Clemmensen reduction, the compounds used are zinc amalgam and hydrochloric acid. Red P means red phosphorus.
Complete answer:
The given reactant on the question is ketone which is a carbonyl compound and it is converted into an alkane. So, we can say that the carbonyl group has been reduced to alkane because there is the addition of hydrogen atoms and removal of oxygen atoms.
Wolff-Kishner reduction is a method in which the given ketone can be converted into an alkane. In Wolff-Kishner reduction, the compounds used are hydrazine, potassium hydroxide, and ethylene glycol. The temperature for this reaction should be around 453-473 K. The reaction is given below:
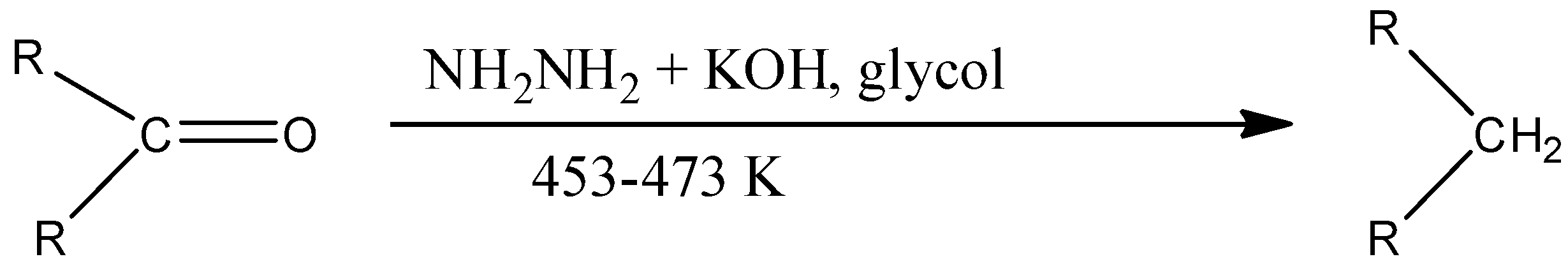
Clemmensen reduction is a method in which the given ketone can be converted into an alkane. In Clemmensen reduction, the compounds used are zinc amalgam which is Zn-Hg, and hydrochloric acid. The reaction is given below:
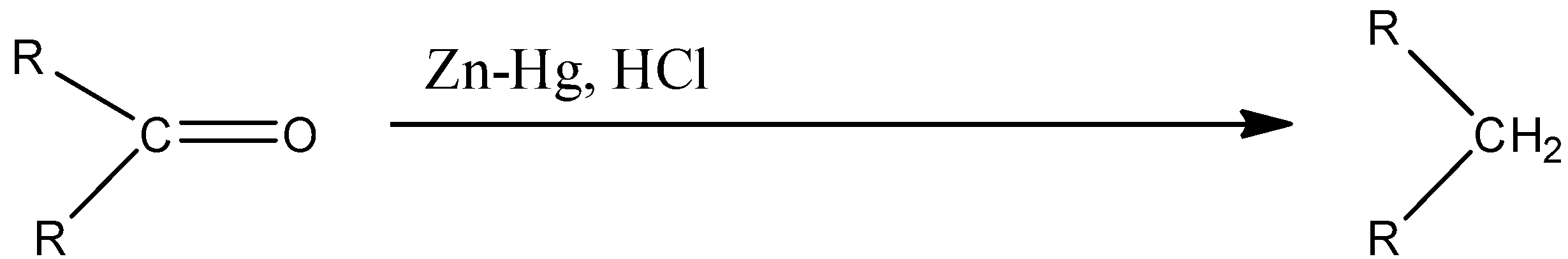
The given ketone can also be converted into the corresponding alkane by using the red phosphorus and hydriodic acid, i.e., HI by keeping the temperature of the reaction around 423-473 K. The reaction is given below:
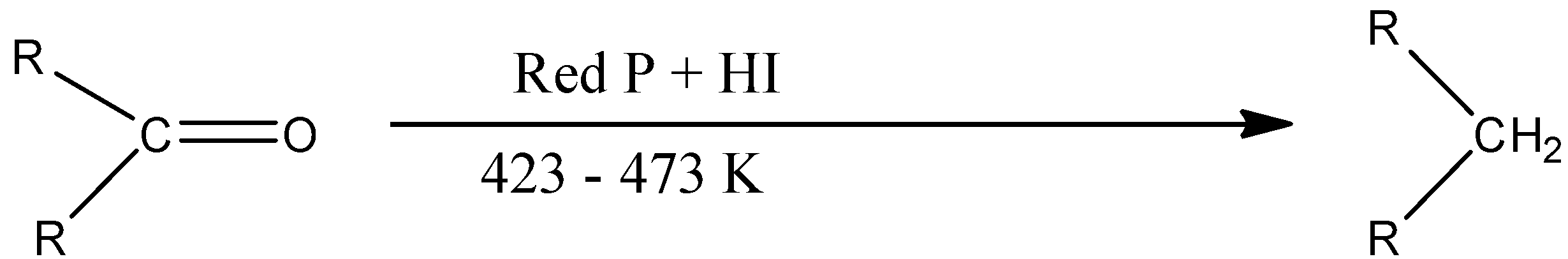
Since all the methods are suitable for converting the ketone to the alkane by all the methods mentioned above.
Therefore, the correct answer is an option (d)- All of these.
Note:
All the reactions mentioned above, i.e., Wolff-Kishner reduction, Clemmensen reduction, Red P, and HI can reduce the given aldehydes to the corresponding alkanes. Clemmensen reduction is used for the carbonyl compounds that are sensitive to alkalies, while the Wolff-Kishner reduction is used for the carbonyl compounds that are sensitive to acids.
