Question
Question: \({O^ - }{O^ - }NH_3^ + \) The high melting point and insolubility of sulphanilic acid in organic...
O−O−NH3+
The high melting point and insolubility of sulphanilic acid in organic solvents are due to:
A.Non-polar nature
B.Zwitter ion structure
C.Van der Waals’ forces
D.None of the above
Solution
The high melting point and insolubility in organic solvents of sulphanilic acid are due to its Zwitterion structure. Every zwitterion has an isoelectric point. You can calculate the isoelectric point of an acid by taking the average of the pKa’s of the two functional groups. Zwitterion, also known as inner salt or dipolar ion.
Complete step by step answer:
Zwitterions have a high melting point and insolubility in organic solvents of sulphanilic acid. Sulphanilic acid acts as zwitterions. It contains amino group and sulfonic acid group and this group is protonated to form cation and the sulphonic acid is deprotonated to form anion.
Zwitterion does not exist as o- and p-amino benzoic acids.
Zwitterion is contained by the high melting point and insolubility of sulphanilic acid in organic solvents.
Structure of Zwitterion sulphanilic acid is:
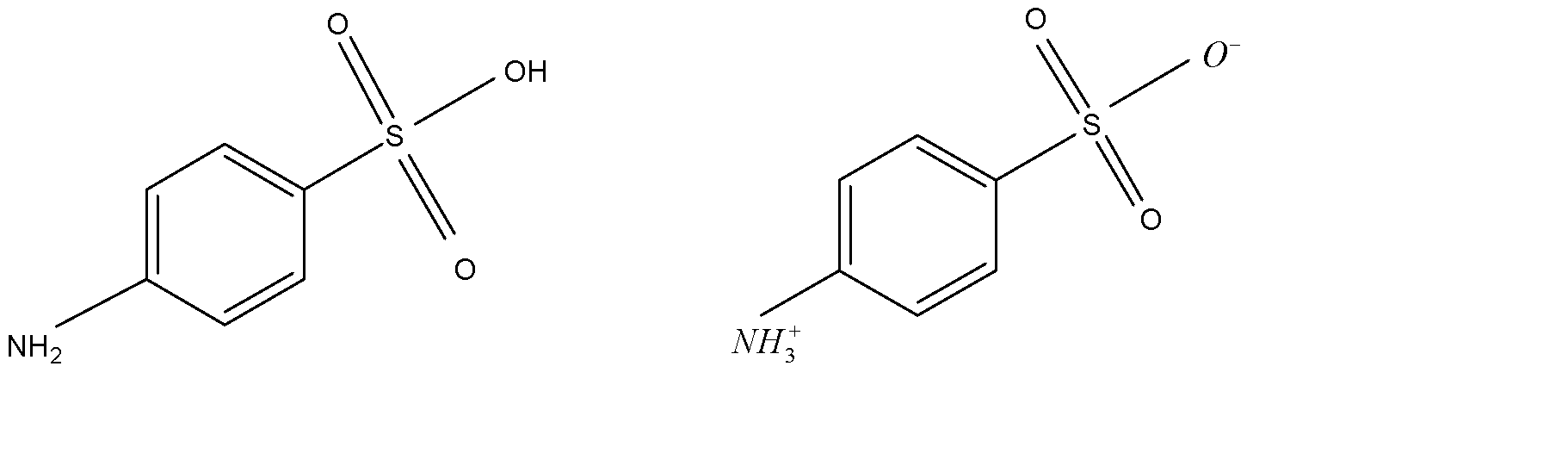
Sulphanilic acid has a high melting point and insolubility in organic solvents are formed by the Zwitterions.
Thus, option (B) is the correct answer.
Additional information:
Sulphanilic acid is an off-white crystalline solid. It is used as an intermediate in the production of food dye and pharmaceutical applications, optical brighteners for white paper and for concrete additives.
Note: Zwitterion comes from the German word Zwitter which means hybrid. It can form other molecules called ampholytes, or amphoteric compounds which act as an acid and a base. Here sulphanilic acid is soluble in organic solvent which produces a high melting point and insolubility.
