Question
Question: Nylon-\(6\) is made by caprolactam which is formed from-- A. Cyclohexanone and hydroxyl amino B...
Nylon-6 is made by caprolactam which is formed from--
A. Cyclohexanone and hydroxyl amino
B. Cyclohexanone and hydrazine
C. benzophenone and hydrazine
D. benzophenone and hydroxylamine
Solution
To answer this question we should know the structure of nylon-6 and caprolactam so, we can take an idea to form the structure about its reactant. The caprolactam is a seven membered ring having six carbon and one nitrogen. It has one carbonyl group and one secondary amine group in the ring.
Complete step-by-step answer: When a large number of molecules join in a continuous way to form a long structure, the structure is known as a polymer. In a polymer, a molecule or unit repeats again, and again that’s why the formed structure is known as a polymer because the word ‘poly‘ means many, and ‘mer’ means units. The units which repeat are known as repeating units or monomers.
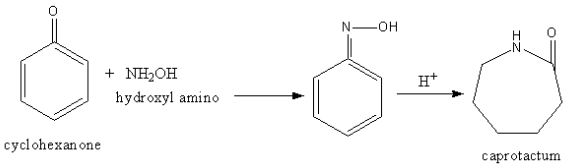
Cyclohexanone and hydroxyl amino react to give caprolactam. The formation of caprolactam is shown as follows:
Cyclohexanone reacts with hydroxyl amino so, a water molecule removes forming a C=N double
bond. Then in acidic medium caprolactam forms.
The caprolactam is allowed to react at high temperatures in a vacuum. The water is used as the initiator. The reaction is shown as follows:
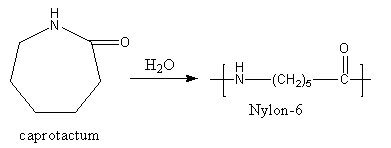
So, nylon-6 is made by caprolactam which is formed from cyclohexanone and hydroxyl amino.
Therefore, option (A) Cyclohexanone and hydroxyl amino, is correct.
Note: Nylon-6 is formed by ring-opening polymerization. Nylon-6 and Nylon-66 , both are different polymers. Nylon-6 is formed by one type of monomer caprolactam. The Nylon-66 is formed by two types of repeating units, hexamethylenediamine, and adipic acid. The number 6 represents the number of carbon atoms. The caprolactam has 6 carbon atoms. Both monomers of Nylon- 66 have 6 carbon atoms.
