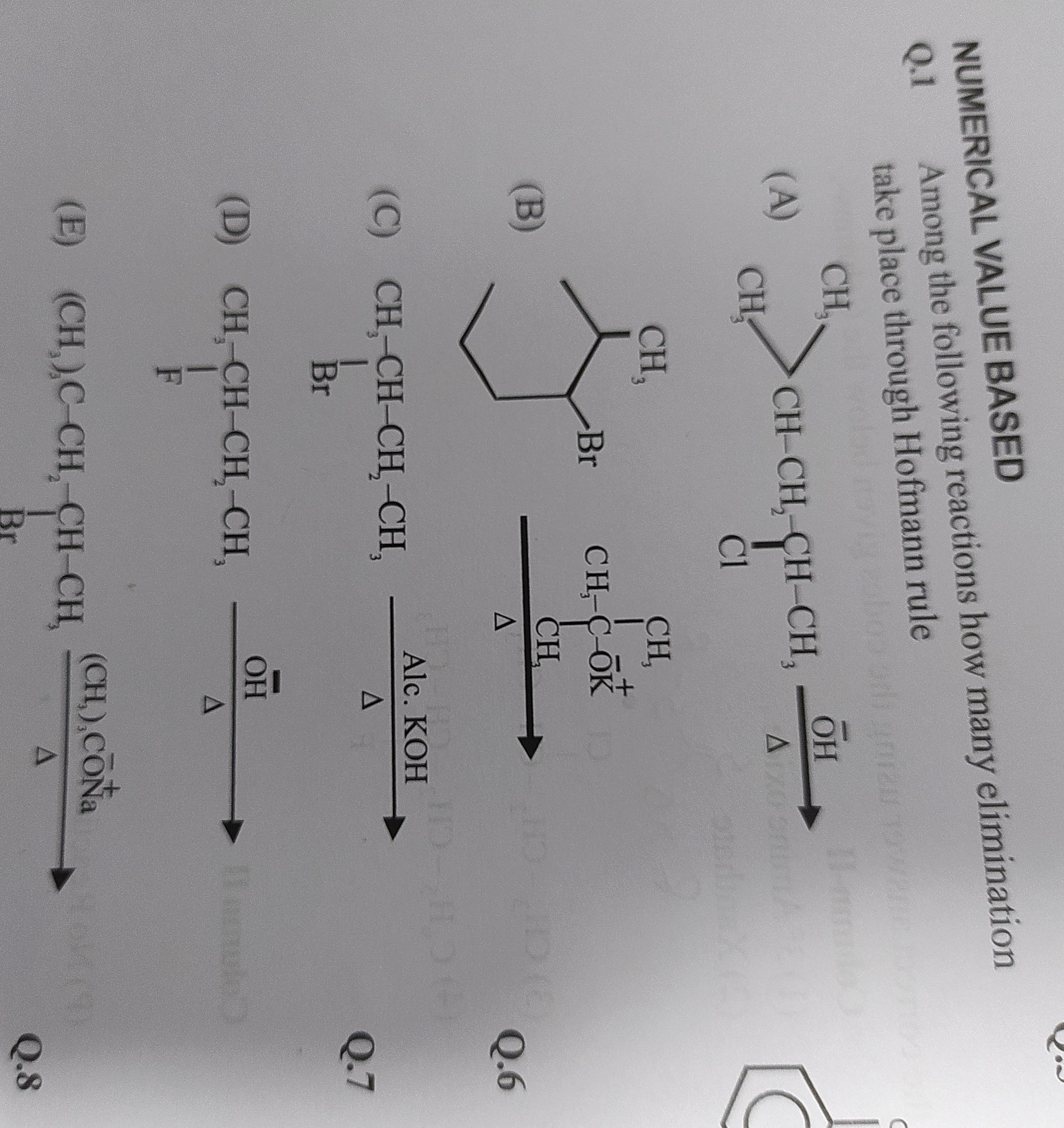
Question
Question: Among the following reactions how many elimination take place through Hofmann rule (A) $CH_3-CH_2-C...
Among the following reactions how many elimination take place through Hofmann rule
(A) CH3−CH2−CH−CH3OH−Δ
(B)
CH3−C+−OKΔ CH3
(C) CH3−CH−CH2−CH3Alc.KOHΔ ∣ Br
(D) CH3−CH−CH2−CH3OH−Δ ∣ F
(E) (CH3)3C−CH−CH2−CH3(CH3)3CONa+Δ ∣ Br
Answer
3
Explanation
Solution
Hofmann's rule states that in an elimination reaction, the major product is the least substituted alkene (Hofmann product). This typically occurs under specific conditions:
- Bulky base: A sterically hindered base preferentially abstracts the least sterically hindered beta-hydrogen, leading to the less substituted alkene.
- Poor leaving group: When the leaving group is poor (e.g., F, or a quaternary ammonium ion), the transition state often has significant carbanionic character, favoring the abstraction of the most acidic proton, which frequently leads to the less substituted alkene.
Let's analyze each reaction:
(A) CH3−CH2−CH(Cl)−CH3OH−Δ
- Reactant: 2-chlorobutane
- Base: OH− (hydroxide ion) is a strong base but not sterically bulky.
- Leaving group: Cl (chloride) is a good leaving group.
- Possible products:
- Elimination from CH3 (C1): But-1-ene (disubstituted, Hofmann product).
- Elimination from CH2 (C3): But-2-ene (trisubstituted, Zaitsev product).
- Conclusion: With a non-bulky base and a good leaving group, the reaction primarily follows Zaitsev's rule, favoring the more substituted alkene (But-2-ene).
- Does NOT follow Hofmann rule.
(B) 1-bromo-2-methylcyclohexane (CH3)3CO−K+Δ
- Reactant: 1-bromo-2-methylcyclohexane
- Base: (CH3)3CO−K+ (Potassium tert-butoxide) is a very bulky base.
- Leaving group: Br (bromide) is a good leaving group.
- Possible products:
- Elimination from C2 (beta-H on C2, adjacent to CH3): Forms 2-methylcyclohexene (trisubstituted alkene, Zaitsev product).
- Elimination from C6 (beta-H on C6): Forms 1-methylcyclohexene (disubstituted alkene, Hofmann product).
- Conclusion: Due to the bulky base, the Hofmann product (1-methylcyclohexene) is favored.
- FOLLOWS Hofmann rule.
(C) CH3−CH(Br)−CH2−CH3Alc.KOHΔ
- Reactant: 2-bromobutane
- Base: Alc. KOH (alcoholic potassium hydroxide) is a strong base but not sterically bulky.
- Leaving group: Br (bromide) is a good leaving group.
- Possible products:
- Elimination from CH3 (C1): But-1-ene (disubstituted, Hofmann product).
- Elimination from CH2 (C3): But-2-ene (trisubstituted, Zaitsev product).
- Conclusion: With a non-bulky base and a good leaving group, the reaction primarily follows Zaitsev's rule, favoring the more substituted alkene (But-2-ene).
- Does NOT follow Hofmann rule.
(D) CH3−CH(F)−CH2−CH3OH−Δ
- Reactant: 2-fluorobutane
- Base: OH− (hydroxide ion) is a strong base but not sterically bulky.
- Leaving group: F (fluoride) is a poor leaving group due to the strong C-F bond.
- Possible products:
- Elimination from CH3 (C1): But-1-ene (disubstituted, Hofmann product).
- Elimination from CH2 (C3): But-2-ene (trisubstituted, Zaitsev product).
- Conclusion: When the leaving group is poor (like F), the Hofmann product (But-1-ene) is favored.
- FOLLOWS Hofmann rule.
(E) (CH3)3C−CH(Br)−CH2−CH3(CH3)3CONa+Δ
- Reactant: 3-bromo-2,2-dimethylbutane
CH3 | CH3 - C - CH(Br) - CH2 - CH3 | CH3 - Base: (CH3)3CONa+ (Sodium tert-butoxide) is a very bulky base.
- Leaving group: Br (bromide) is a good leaving group.
- Possible products:
- Elimination from CH2 (adjacent to C(Br)): Forms 3,3-dimethylbut-1-ene (disubstituted alkene, Hofmann product).
CH3 | CH3 - C - CH = CH2 | CH3 - Elimination from one of the CH3 groups of the tert-butyl group (adjacent to C(Br)): Forms 2,3-dimethylbut-2-ene (tetrasubstituted alkene, Zaitsev product).
CH3 | CH3 - C = C - CH2 - CH3 | CH3
- Elimination from CH2 (adjacent to C(Br)): Forms 3,3-dimethylbut-1-ene (disubstituted alkene, Hofmann product).
- Conclusion: Due to the bulky base, the Hofmann product (3,3-dimethylbut-1-ene) is favored.
- FOLLOWS Hofmann rule.
Therefore, reactions (B), (D), and (E) take place through Hofmann's rule. The total number of such reactions is 3.
