Question
Question: Number of stereoisomerism possible for given compound 2-bromo-3-chlorobutane ...
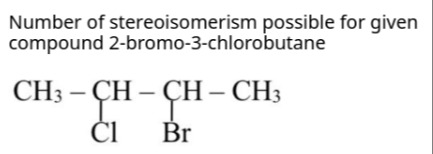
Number of stereoisomerism possible for given compound 2-bromo-3-chlorobutane
4
Solution
To determine the number of stereoisomers for 2-bromo-3-chlorobutane, we first need to identify the chiral centers in the molecule.
-
Draw the structure of 2-bromo-3-chlorobutane:
The parent chain is butane (4 carbons).
The substituents are a bromine at position 2 and a chlorine at position 3.
According to IUPAC rules, when numbering, the substituents are given the lowest possible locants, and if there's a tie, alphabetical order is used. 'Bromo' (B) comes before 'chloro' (C). So, bromine should get the lower number.
The structure is:
CH₃ - CH(Br) - CH(Cl) - CH₃ -
Identify chiral centers:
A chiral center is a carbon atom bonded to four different groups.- Carbon 1 (CH₃): Not a chiral center.
- Carbon 2 (CH(Br)): A chiral center.
- Carbon 3 (CH(Cl)): A chiral center.
- Carbon 4 (CH₃): Not a chiral center.
So, there are two chiral centers in 2-bromo-3-chlorobutane (n=2).
-
Determine the number of stereoisomers:
The maximum number of stereoisomers for a compound with 'n' chiral centers is given by the formula 2n.
This formula applies when the molecule is unsymmetrical, meaning it does not possess an internal plane of symmetry that would lead to a meso compound.In this case, the two chiral centers are different (one has a bromine atom and the other has a chlorine atom). This means the molecule is unsymmetrical. There is no possibility of a meso compound.
Therefore, the total number of stereoisomers = 2n=22=4.
