Question
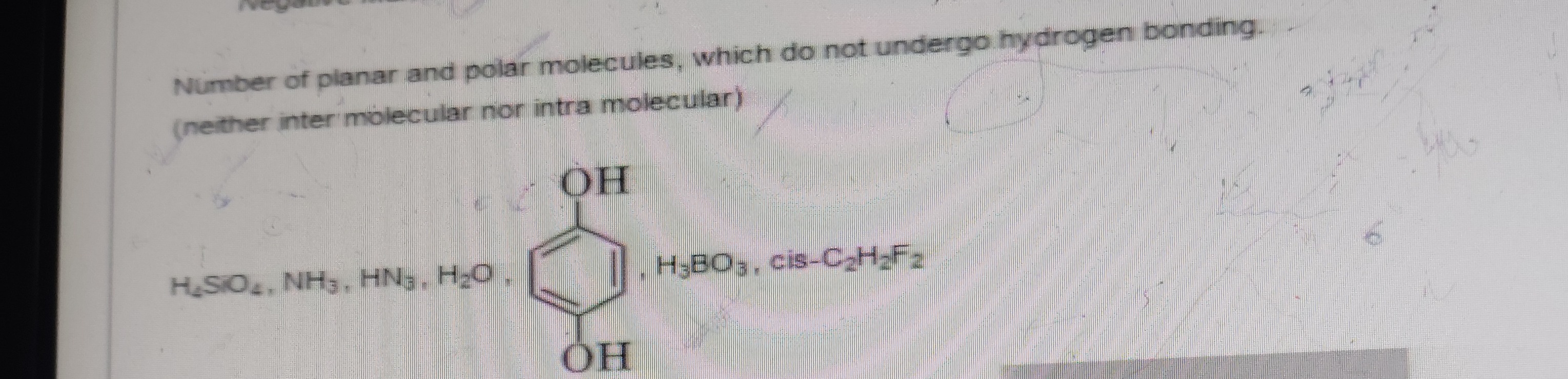
Question: Number of planar and polar molecules, which do not undergo hydrogen bonding. (neither inter molecula...
Number of planar and polar molecules, which do not undergo hydrogen bonding. (neither inter molecular nor intra molecular)
H2SO4,NH3,HN3,H2O,H3BO3,cis−C2H2F2
Answer
1
Explanation
Solution
The given molecules are H2SO4,NH3,HN3,H2O,H3BO3,cis−C2H2F2. We need to find the number of planar and polar molecules from this list which do not undergo hydrogen bonding (neither intermolecular nor intramolecular).
Let's analyze each molecule:
- H2SO4: The central sulfur atom is sp3 hybridized, with a tetrahedral electron geometry. The molecule has a distorted tetrahedral molecular geometry. Thus, it is not planar. It is polar and undergoes intermolecular hydrogen bonding.
- NH3: The central nitrogen atom is sp3 hybridized, with a tetrahedral electron geometry and trigonal pyramidal molecular geometry. Thus, it is not planar. It is polar and undergoes intermolecular hydrogen bonding.
- HN3: Hydrazoic acid (HN3) has a bent structure at the first nitrogen atom (bonded to H). The N-N-N part is linear, but the H-N-N angle is approximately 112 degrees. Thus, the molecule is not planar. It is polar and undergoes intermolecular hydrogen bonding through the N-H bond.
- H2O: Water has a bent molecular geometry, which lies in a plane. Thus, a water molecule is planar. It is polar and undergoes extensive intermolecular hydrogen bonding.
- H3BO3: Boric acid has a central boron atom which is sp2 hybridized, and the three oxygen atoms bonded to it lie in a plane. The O-H bonds are also in this plane. Thus, the entire molecule is planar. It is polar and undergoes extensive intermolecular hydrogen bonding.
- cis−C2H2F2: In cis-1,2-difluoroethene, the two carbon atoms of the double bond and the atoms bonded to them lie in a plane. Thus, the molecule is planar. The molecule has polar C-F bonds and C-H bonds. Due to the cis arrangement, the bond dipoles do not cancel out, resulting in a net dipole moment. Thus, it is polar. There are no O-H, N-H, or F-H bonds, so it does not undergo hydrogen bonding.
Now, let's filter the molecules based on the given conditions:
- Planar molecules: H2O, H3BO3, cis−C2H2F2.
- Polar molecules among the planar ones: H2O, H3BO3, cis−C2H2F2.
- Molecules among these that do not undergo hydrogen bonding:
- H2O: undergoes hydrogen bonding.
- H3BO3: undergoes hydrogen bonding.
- cis−C2H2F2: does not undergo hydrogen bonding.
Thus, only cis−C2H2F2 satisfies all the conditions. The number of such molecules is 1.
