Question
Question: Number of pi bonds in \[Xe{O_4}\]is:...
Number of pi bonds in XeO4is:
Solution
The structure of xenon compound reveals the number of pi bonds present. The hybridization of xenon compounds determines the number of bonds present in between xenon and oxygen atoms.
Complete step by step answer: XeO4 is the compound formed of xenon and fluorine atoms. Xenon is the central atom in XeO4.
XeO4 is synthesized by treating sodium or barium xenate with conc.H2SO4. Sodium and barium xenates have chemical formulas as Na4XeO6 andBa2XeO6 . The XeO4 is purified using sublimation at195K .
Na4XeO6+2H2SO4→XeO4+2Na2SO4+2H2O
Ba2XeO6+2H2SO4→XeO4+2BaSO4+2H2O
In order to find the number of pi bonds in XeO4, the number of structures has to be determined first. For this the VSEPR theory is used. Xenon is an element in the periodic table with atomic number 54 and electronic configuration is[Kr]4d105s25p6 .
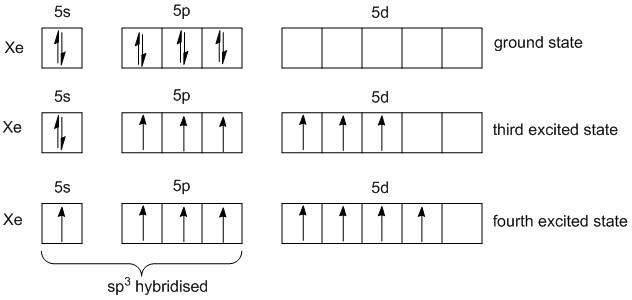
The number of valence electrons of Xenon in ground state is 8. In the fourth excited, xenon atom has 8 unpaired electrons. One electron in s-orbital and three electrons in p-orbitals undergo sp3 hybridization.
As no lone pair of electrons is present the shape of XeO4 is tetrahedral with the bond angle of109o28′. The structure of the XeO4 reveals that there are four sigma bonds and four pi bonds. The s and p orbitals are involved in sigma bonds and the d-orbitals are involved in pi bonds.The pi bonds are formed between the p-orbitals of oxygen and the d-orbitals of xenon. Thus they are also labeled as pπ−dπ bonds.
Note:
Xenon is a noble gas but it forms compounds in excited states. The outer 5d orbitals are used for pi bond formation.
