Question
Question: Number of oxygen atoms attached phosphorus atom in \( {{P}_{4}}{{O}_{6}} \) molecule are: (A) \( 6...
Number of oxygen atoms attached phosphorus atom in P4O6 molecule are:
(A) 6
(B) 4
(C) 3
(D) 2
Solution
Hint : We know that P4O6, is a molecular formula of pentoxide which consists of four molecules of phosphorus and six molecules of oxygen. The phosphorus can make a maximum of five bonds with other molecules whereas oxygen can make a maximum of two bonds with other molecules.
Complete Step By Step Answer:
As we know, the structure of phosphorus pentoxide forms a hexagonal lattice and is held together by the Vander Waal forces. The structure consists of at least four polymorphs. Phosphorus hexoxide P4O6, is a dimer of the compound phosphorus (V) oxide P4O5. It is a covalent compound. Phosphorus is basically a pentavalent element and it reacts with oxygen to form oxides and both of these oxides of phosphorus exist as dimer that means these compounds are formed by a combination of two molecules of that compound. The compound is a very good dehydrating agent. To find out the number of bridging oxygen atoms in P4O6, we need to first understand what a bridging atom means. Further, we need to understand the chemical structure of P4O6. The phosphorus atoms and oxygen atoms bonded to form a closed cage like structure. There are five bonds and each oxygen atom undergoes hybridisation. There are four where hybridisation of oxygen is sp3 .
Thus, No. of oxygen atoms attached with phosphorus = 3
Phosphorus trioxide is the chemical compound with the molecular formula P4O6. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today.
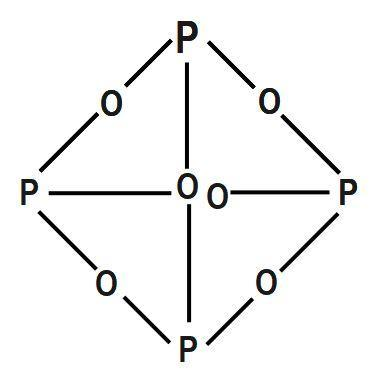
Therefore, the correct answer is option C.
Note :
Remember that students should not confuse the term ‘bridging atom’ with ‘ligand’. Ligand is the term used in coordination chemistry. A ligand can be an ion or molecule and it binds the central metal atom or ion to form coordination complexes. It can be anion or cation or sometimes it can be a neutral molecule.
