Question
Question: Number of nodal planes in \( 4p \) sub-orbit will be: (A) \( 0 \) (B) \( 1 \) (C) \( 2 \) ...
Number of nodal planes in 4p sub-orbit will be:
(A) 0
(B) 1
(C) 2
(D) 3
Solution
On the basis of the values of principal quantum number and azimuthal quantum number, we can determine that in which part of the suborbital, the probability of finding an electron is maximum or minimum.
Complete answer:
Node: It is a point, surface or a plane in which the probability of finding an electron is zero. These are categorized into two parts:
Radial nodes: It is also known as the nodal region and it is a spherical surface around the sub orbitals where the probability of finding electrons is zero. Its value is equal to n−l−1 , where n is the principal quantum number and l is the azimuthal quantum number.
Angular nodes: It is also known as a nodal plane. It is a plane around the nucleus where the chances for finding an electron is zero. Its value is equal to azimuthal quantum number l .
The major concept in quantum mechanics is that the electrons show both wave as well as particle nature and the nodal planes around the nucleus indicate the wave nature of electrons i.e., if there are no electrons present then there would be no amplitude of vibration.
For 4p suborbital,
The value of principal quantum number n=4
So, the value of azimuthal quantum number l lies between 0 to n−1
⇒l=0 to 4−1
⇒l=0 to 3
We know that for p suborbital, l=1
Hence, the number of nodal planes for 4p suborbital =1
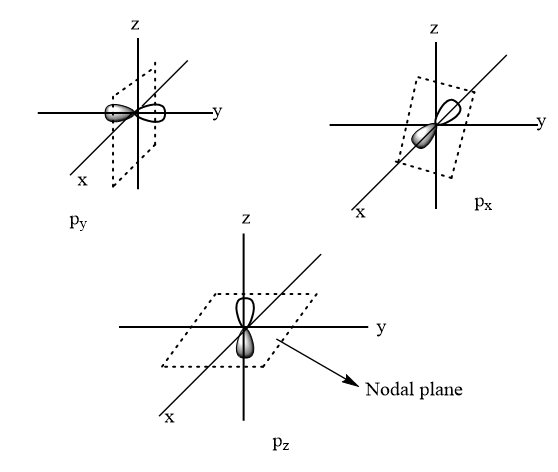
Thus, option (B) is the correct answer.
Note:
It is important to note that the atoms or molecules with few numbers of nodal planes are comparatively stable that those who have large numbers of nodal planes because the atoms with greater nodal planes are very unstable and reactive. Also, the number of nodal planes in a molecule directly varies with the free energy associated with the group of bonded atoms.
