Question
Question: Number of moles of hydrogen atoms required to get one mole of hydrazobenzene from nitrobenzene is: ...
Number of moles of hydrogen atoms required to get one mole of hydrazobenzene from nitrobenzene is:
A. 10
B. 5
C. 8
D. 4
Solution
Nitrobenzene has the chemical formula of C6H5NO2. Whereas hydrazobenzene has two aromatic rings which are attached with an amine group. A number of moles of a substance are the ratio of the mass to the molecular mass of the compound.
Complete Solution:
-The process of formation of hydrazobenzene includes a total of five steps in which the reduction of every compound takes place.
-Firstly, the nitrobenzene is reduced, in which the 2 hydrogen atom replaces the oxygen atom from the nitro group and forms the nitroso benzene.
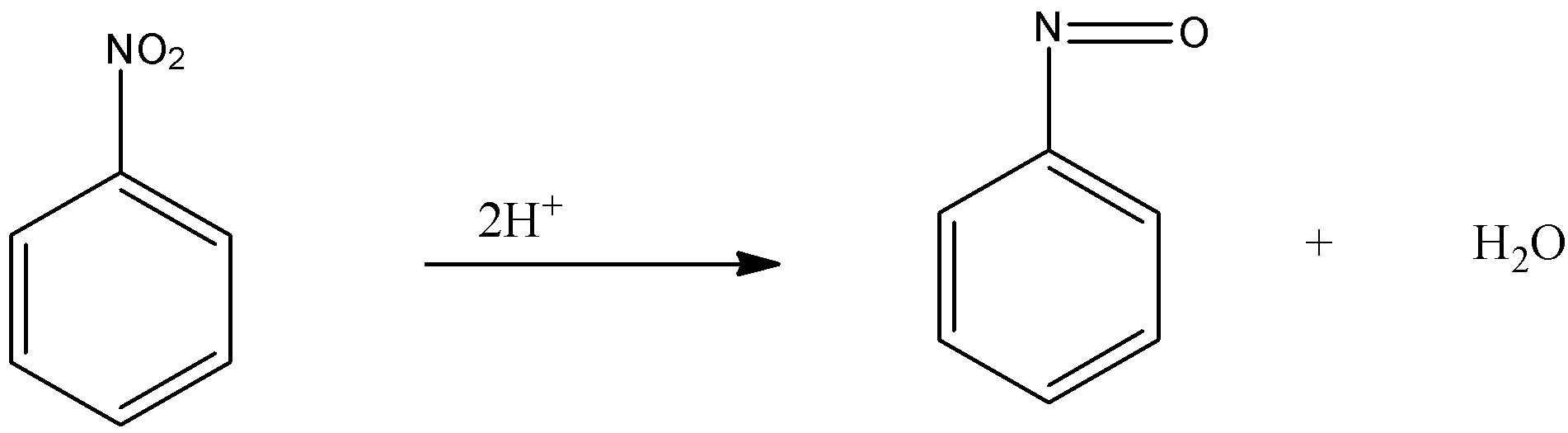
-Now, this nitroso benzene is again reduced and the oxygen atom present with the nitrogen group is replaced by the 4 hydrogen and forms N- phenylhydroxylamine.
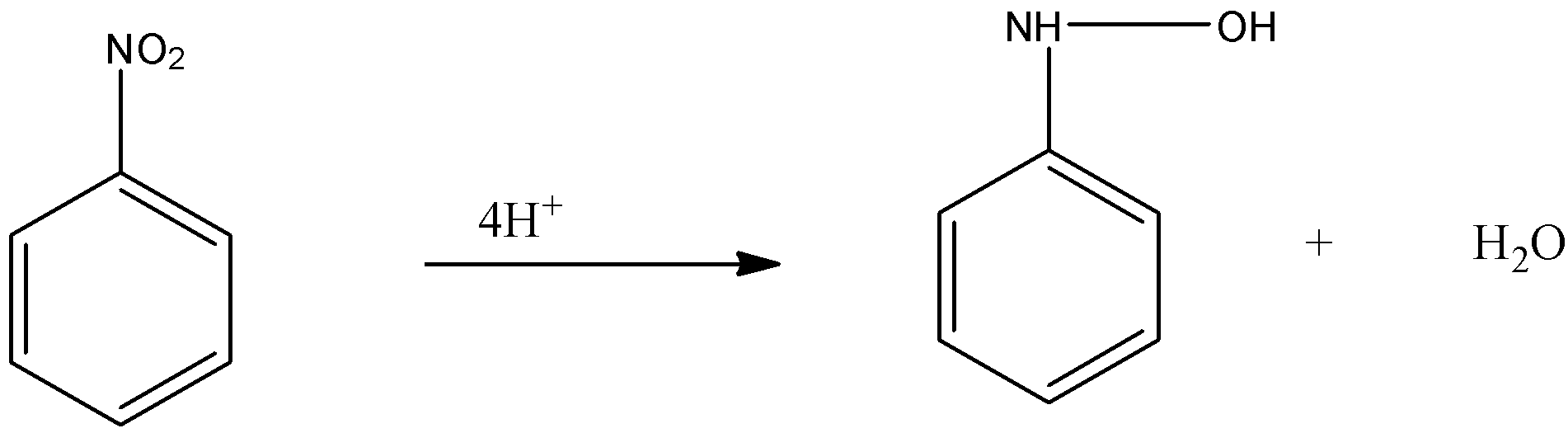
-Now, the N- phenylhydroxylamine and nitroso benzene combine to form azoxybenzene after which it undergoes reduction with the help of 2 hydrogen atoms and forms azo benzene i.e.
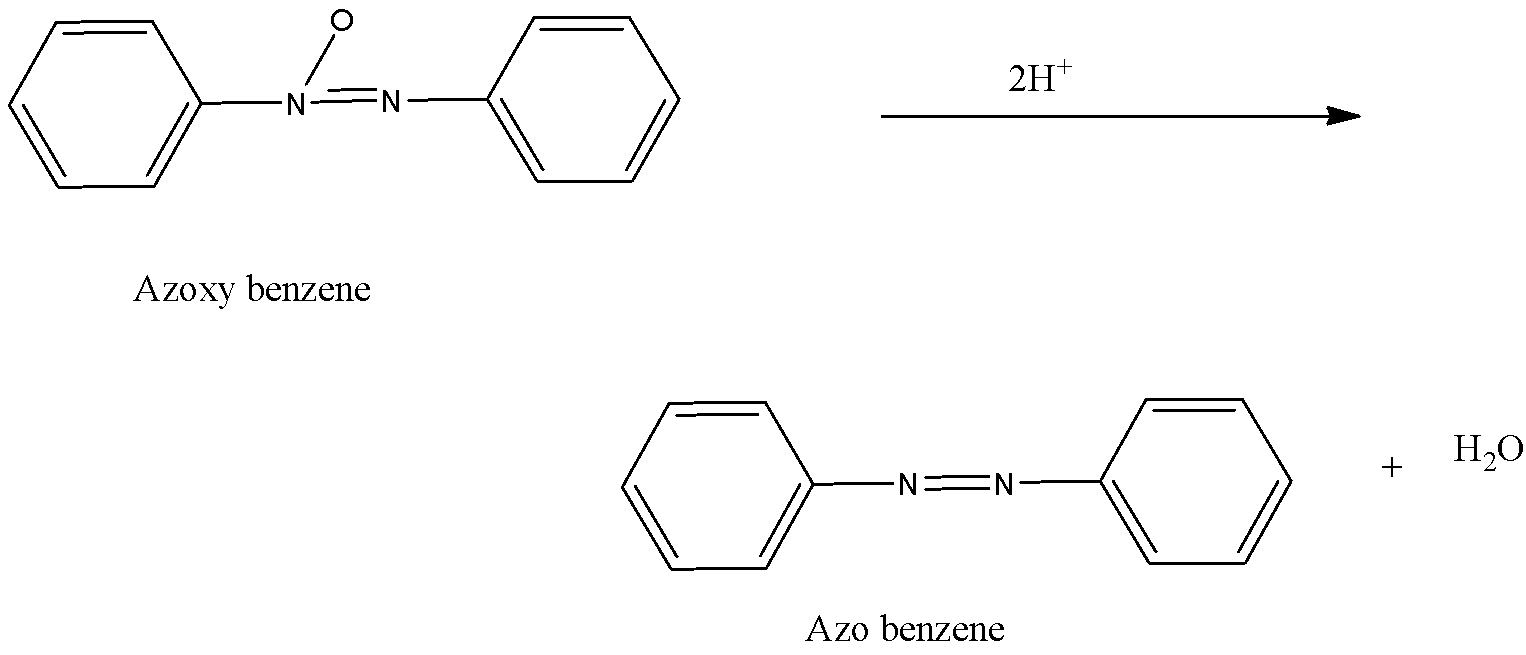
-Now, in the final step, the azobenzene is again by the introduction of two hydrogen atoms on the nitrogen. The double bond present between nitrogen atoms break and the attachment of hydrogen atom take place and the structure is known as hydrazo benzene.
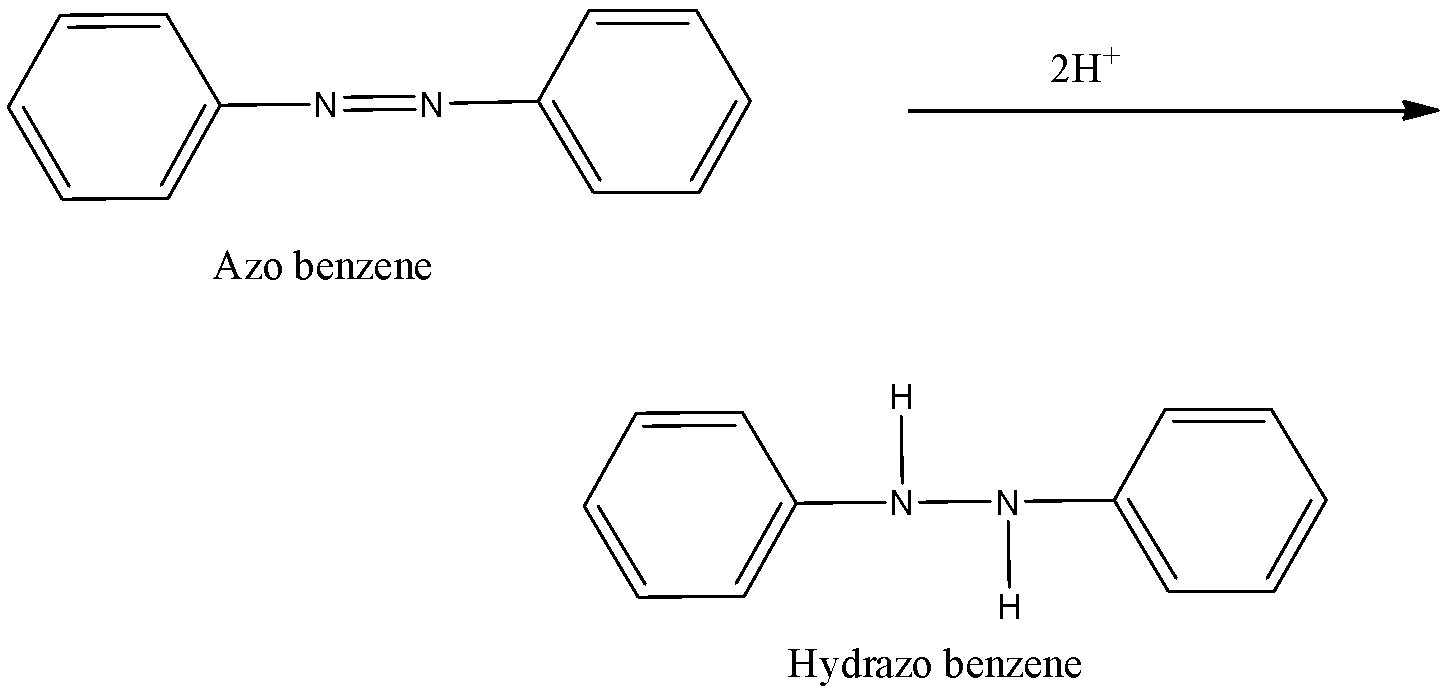
-So, from the above process, we can calculate the no. of moles of hydrogen atoms that were responsible for the reduction process that is 10 (2 + 4 + 2 + 2).
So, the correct answer is “Option A”.
Note: Nitrobenzene can also be used in the synthesis of aniline. Aniline is the 6 membered ring structure with an amine group attached to it and has a molecular formula of C6H5NH2. Azobenzene can also be produced with the help of LiAlH4 that is a strong reducing agent.
