Question
Question: Number of isomers of hexane...
Number of isomers of hexane
Solution
Hint: To correctly tell the answer of the question, we should know that isomers are molecules that have the same molecular formula, but have a different arrangement of the atoms in space.
Step by step solution:
We should know that Hexane is a straight-chain alkane with six carbon atoms and has the molecular formula C6H14. We should also know that isomers have the same molecular formula, but have a different arrangement of the atoms in space. We should know that in structural isomerism, the atoms are arranged in a completely different order.
Now, we will draw isomers of hexane. We should follow these important points to draw correct isomers. We should first identify and count all the atoms which are to be drawn in the isomers. This will give us a molecular formula. We should know that any isomers drawn will contain the same number of each type of atom found in the original molecular formula of the compound. So, let us draw the isomers of hexane.
| Common name | IUPAC name | Text formula | Skeletal formula |
|---|---|---|---|
| normal hexane n-hexane | hexane | CH3CH2CH2CH2CH2CH3 | 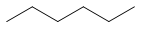 |
| Iso-hexane | 2-methylpentane | (CH3)2CH(CH2)2CH3 | 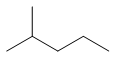 |
| 3-methylpentane | CH3CH2CH(CH3)CH2CH3 | 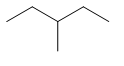 | |
| 2,3-dimethylbutane | (CH3)2CHCH(CH3)2 | 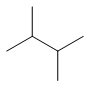 | |
| neohexane | 2,2-dimethylbutane | (CH3)3CCH2CH3 | 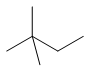 |
Note:
We use hexanes in industries for formation of glues for shoes, leather products. We use hexane to extract oils from seeds, for cleansing and degreasing a variety of items, and in textile manufacturing. We should know that hexanes are chiefly obtained by refining crude oil. It is important to note that like most alkanes, hexane characteristically exhibits low reactivity and are suitable solvents for reactive compounds. We should note that acute (short-term) inhalation exposure of humans to high levels of hexane causes mild central nervous system (CNS) effects, including dizziness, giddiness, slight nausea, and headache.
