Question
Question: Number of ionisable and non ionisable \(C{l^ - }\) ions in \(CoC{l_3}.5N{H_3}\) respectively are, if...
Number of ionisable and non ionisable Cl− ions in CoCl3.5NH3 respectively are, if one mole of it reacts with two mole of silver nitrate completely and forms ppt.
A.3,0
B.2,1
C.1,2
D.0,3
Solution
CoCl3.5NH3 is a coordination complex. This complex reacts with silver nitrate and precipitates. The number of ionizable and non ionizable ions depends on the type of valency in a complex.
Complete step by step answer:
-The IUPAC name of CoCl3.5NH3 is pentaamminechlorocobalt (III) chloride.
-The structure of CoCl3.5NH3 is given below as follows:
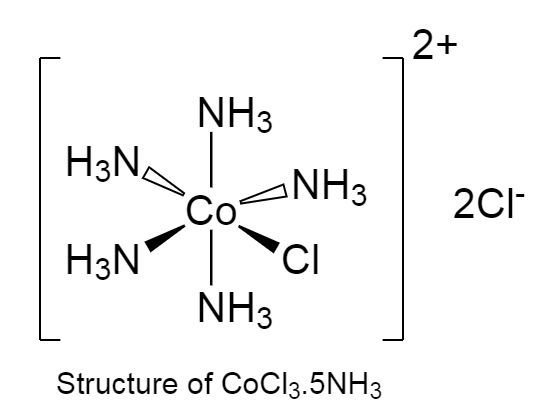
-In the given coordination complex, five NH3 molecule are directly attached to cobalt metal.
-Only one chloride ion is attached to cobalt metal.
-The rest of the chloride ion is satisfying the oxidation state of the metal.
-The chloride ion that is satisfying the oxidation of metal is called primary valency.
-And the one that is attached directly to metal is called secondary valency.
-Primary valency consists of ionisable ions whereas secondary valency consists of non ionizable ions.
-Primary valencies are satisfied by the negative charges whereas secondary valencies are satisfied by the positive charge or neutral species.
-The number of secondary valence shown by the metal is fixed.
-Thus, therefore the formula for the complex is [Co(NH3)5Cl]Cl2 .
-One mole of CoCl3.5NH3 reacted with two moles of silver nitrate to form precipitate.
-Since the primary valency of chloride ions in the complex is two, therefore the number of ionisable ions will also be.
-Similarly for non ionizable ions, it is the same as secondary valency that is one.
-Thus, the number of ionizable ions is two and non ionisable ions is one.
So, the correct answer is option B) 2,1 .
Note: Primary valency is the number of ions that satisfies the charge of the ion. Secondary valency is the same as the coordination number of a complex. In the structure of the complex, the primary valency is represented by a full line whereas the secondary valency is represented by a dotted line.
