Question
Question: Number of geometrical isomers of \({\left[ {Pt\left( {NH_3} \right)\left( {NO_2} \right)Py\left( {NH...
Number of geometrical isomers of [Pt(NH3)(NO2)Py(NH2OH)]+ is
A. 2
B. 3
C. 4
D. 5
Solution
In the given question we have to recognize the isomeric structures of the given complex of geometrical form. When a restricted rotation is present somewhere in a molecule it is said to be a geometrical isomerism and the compound is called a geometrical isomer.
Complete step by step solution:
Isomers are those molecules which have the same molecular formula whereas have a different array of the atoms in space. That contains any different arrangements which are simply due to the rotation of molecules as a whole, or rotation of particular bonds.
In the given complex [Pt(NH3)(NO2)Py(NH2OH)]+ Pt is the central atom and is connected with four different ligand or we call groups therefore it will have 3 different structure according to their functional group attached to it. These are known to be their geometrical isomers of the complex.
Therefore, there are 3 geometrical isomers of the given complex.
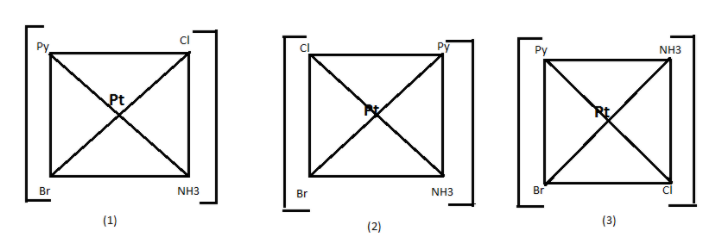
Hence, option (B) is a correct answer.
Note: Geometric isomerism is also known as cis-trans isomerism or E-Z isomerism. The geometrical isomers occur where they have a restricted rotation somewhere in a molecule. To recognize the possibility of a geometric isomerism we obviously need to detect or have a restricted rotation somewhere in the complex of a molecule.
