Question
Question: Number of equatorial bond in \[PC{l_5}\]is...
Number of equatorial bond in PCl5is
Solution
Hint : We must know that the axial bond is longer than the equatorial bond because of greater repulsion from the equatorial bonds.
Complete step by step solution :
We must know that the bonds in an element to non-ring atoms are termed as axial or equatorial depending on their bond angle. The bonds to non-ring atoms with the angles of about 90° to that ring plane are termed as axial, whereas bonds to non-ring atoms which make only the small angle which is compared with the plane of the ring is termed as equatorial.
In Phosphorus pentachloride (PCl5), there are 2 (two) axial bonds and 3 (three) equatorial bonds. The 2-axial bonds are at 90o angle to the 3-equatorial bonds while all other equatorial bonds are at 120o angle to each other atoms. So this axial bond is nearer to the equatorial bond and this causes a greater repulsion which then results in elongation of the bond length.
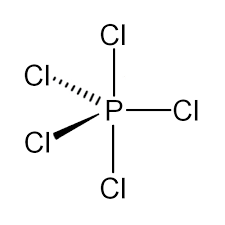
Therefore, we can conclude that the number of equatorial bonds in PCl5 is three.
Additional information: we must understand that the PCl5 has the trigonal bipyramidal structure. The hybridization is sp3dwhich are hybrid orbitals occupied singly with Bond angles of 90o and 120o.
Note : We must also know that inPCl5, the axial bond suffers repulsion from the equatorial bond so that they become weak and long but in PF5the equatorial and axial bonds are of approx. nearly equal in length because the repulsion between these bonds tries to make axial bonds longer but fluorine being the most electronegative makes the above P-F bond shorter as a result of which axial bonds become nearly equal to the equatorial bonds.
