Question
Question: Number of double bond present in the Lewis structure of \( PF_6^ - \) is :...
Number of double bond present in the Lewis structure of PF6− is :
Solution
For hexafluorophosphate, [ PF6 ]-, there are six bonded groups and so no lone pairs. This anion is useful in synthesis as it also aids the crystallisation of bulky cations by having a suitable size match for the cation. The ion's negative charge is distributed through all seven atoms.
Complete answer:
The valence shell electron pair repulsion (VSEPR) theory is a mathematical model for predicting 3-D molecular geometry based on the number of valence shell electron bond pairs between atoms in a molecule or ion. As long as the central atom is not a metal, it can predict the form of almost all compounds with a central atom.
In this compound the central atom is phosphorus. We know that initially the valence electron on the central atom is 5 . The 6 Fluoride atom contributes one electron each. Hence, we add one negative charge to P. So the total valence electron is 12 .
Dividing 12 by 2 , we get 6 . Hence, the geometry formed in 6 electron pairs is Octahedral geometry. Therefore, The VSEPR model for [PF6]− is Octahedral.
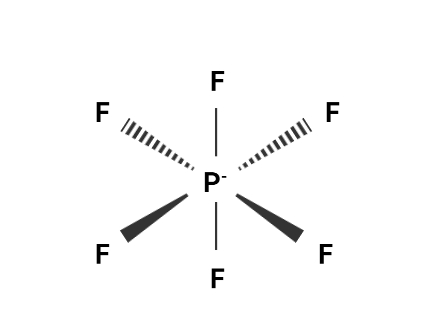
In this figure, we can conclude that there are no double bonds in the compound. Hence, the No. of double bonds present in the Lewis structure of PF6− is 0 .
Note:
Hexafluorophosphate is most commonly used as the lithium salt, lithium hexafluorophosphate. This salt is a natural electrolyte in industrial secondary batteries, such as lithium-ion cells, when combined with dimethyl carbonate.
