Question
Question: Number of compounds which can give Haloform reaction: 
Solution
Haloform reaction is a chemical reaction that is used to detect the presence of −COCH3 group in the molecule. If the −CO group that is connected to the methyl group is connected to another oxygen atom then the compound will not give the haloform reaction.
Complete answer:
Haloform reaction is a chemical reaction in which the haloform like chloroform, bromoform, or iodoform is produced when the organic compound is treated with halogens like chlorine, bromine, or iodine and a base. The compounds that have −COCH3 group in the molecule, only those compounds will give the haloform reaction. If the −CO group that is connected to the methyl group is connected to another oxygen atom then the compound will not give the haloform reaction. In a haloform reaction, there is a formation of precipitate that indicates the presence of −COCH3 group.
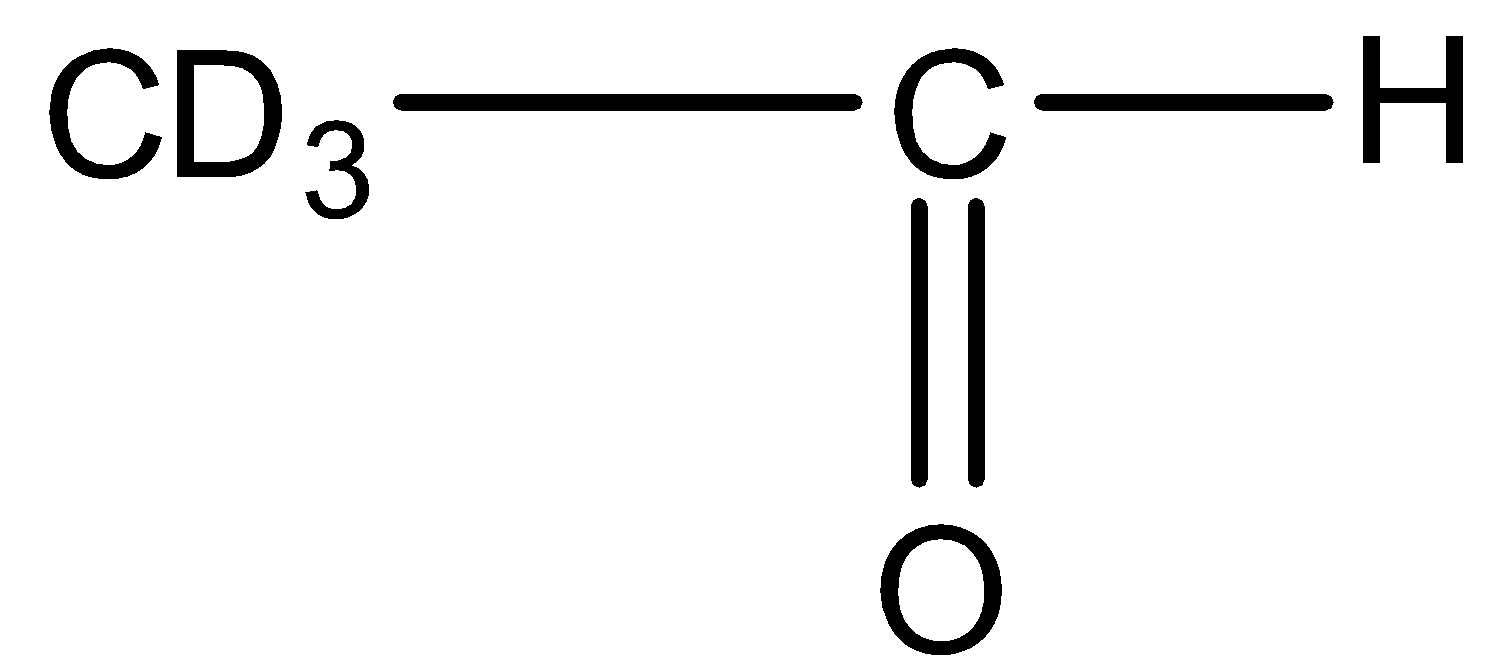
This will give the haloform reaction because there is a −COCD3 group and the −CO group is not attached to any other oxygen atom. D is the isotope of hydrogen so it will give the haloform reaction.
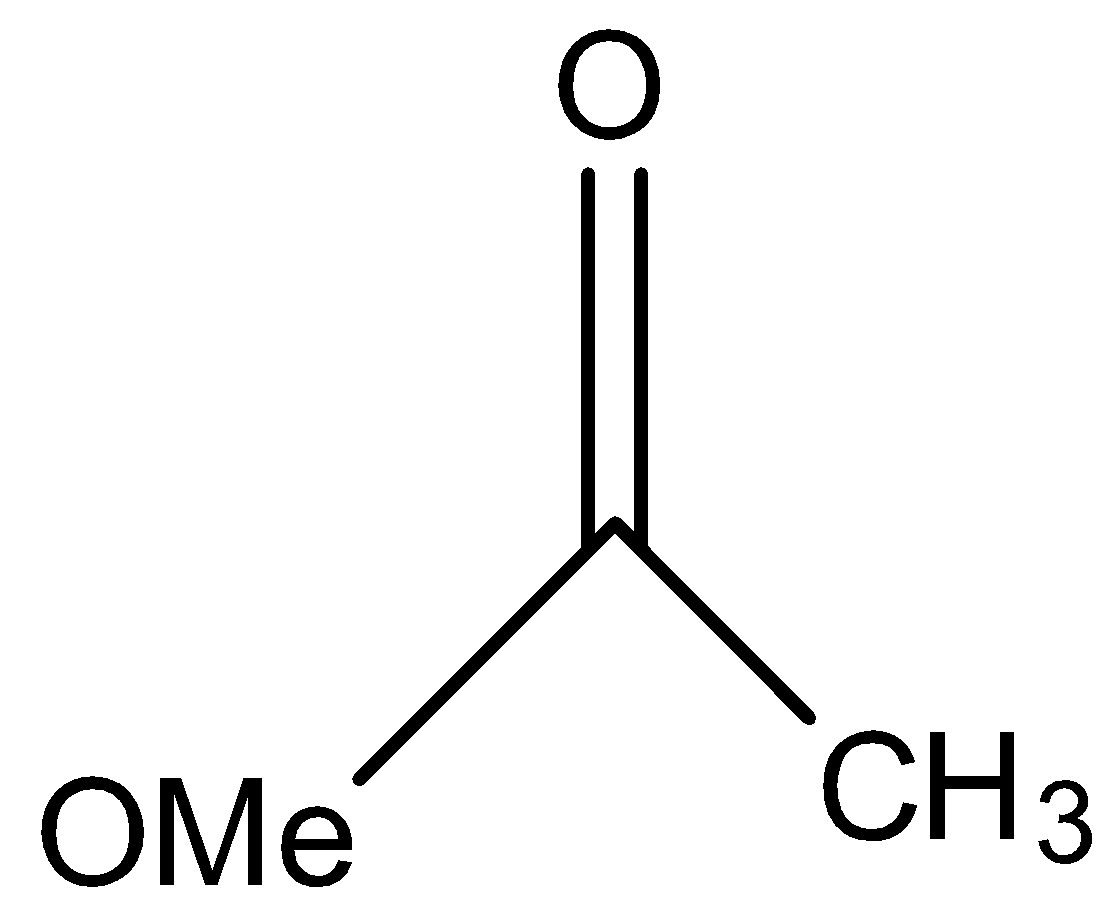
This compound will not give the haloform reaction because the −COCH3 group is attached to another oxygen atom.
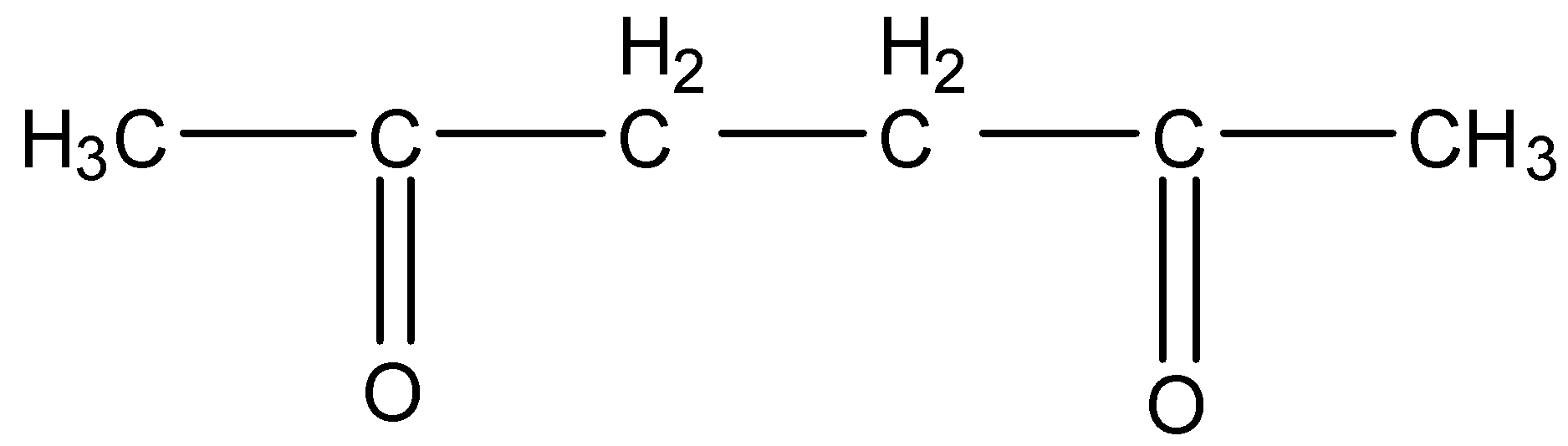
This will give the haloform reaction because there is −COCH3 group and the −CO group is not attached to any other oxygen atom.
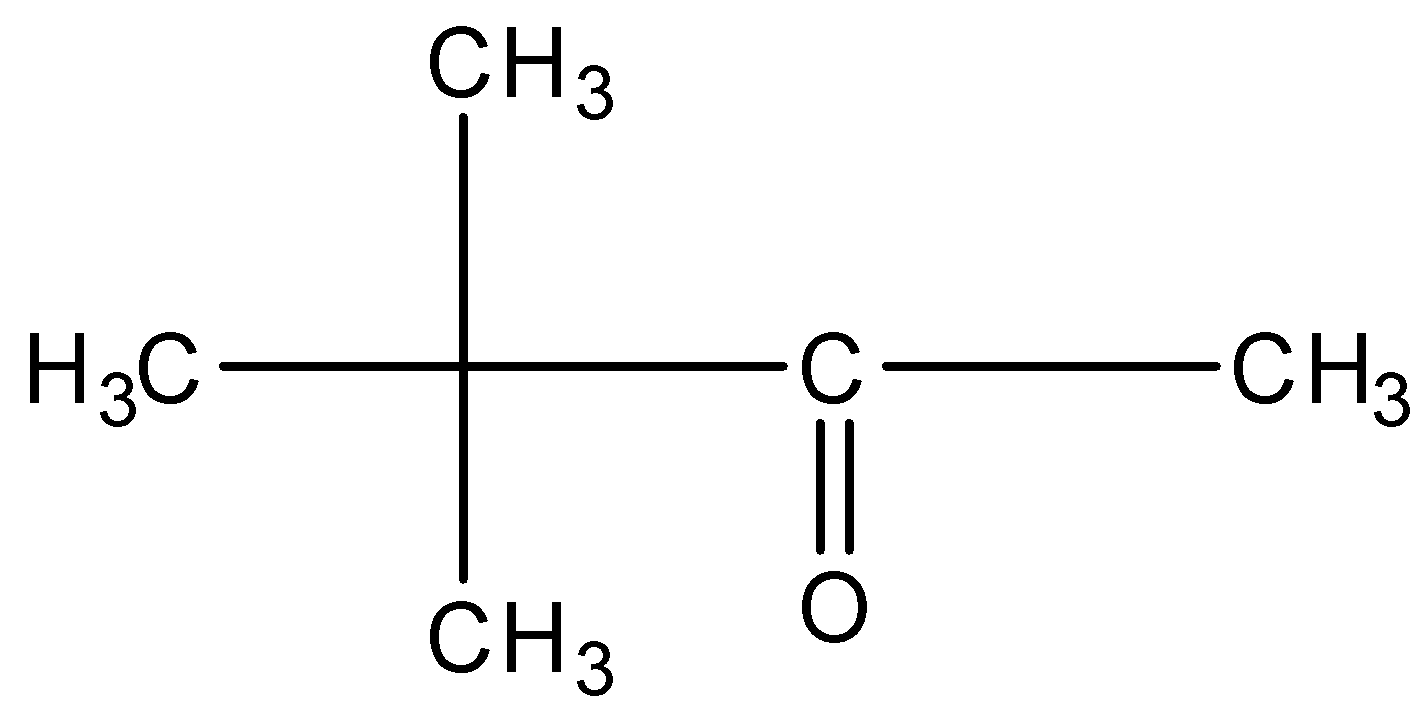
This will give the haloform reaction because there is a −COCH3 group and the −CO group is not attached to any other oxygen atom.
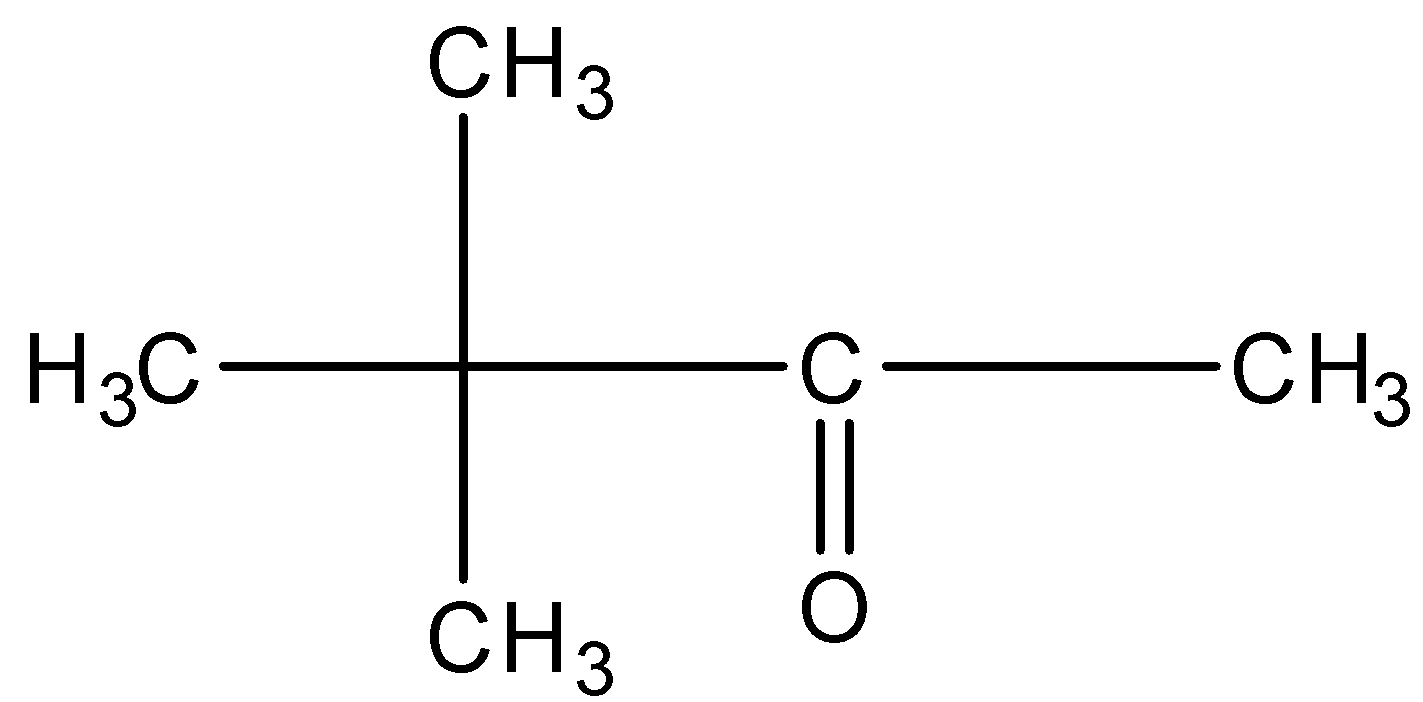
This will give the haloform reaction because there is a −COCH3 group and the −CO group is not attached to any other oxygen atom.
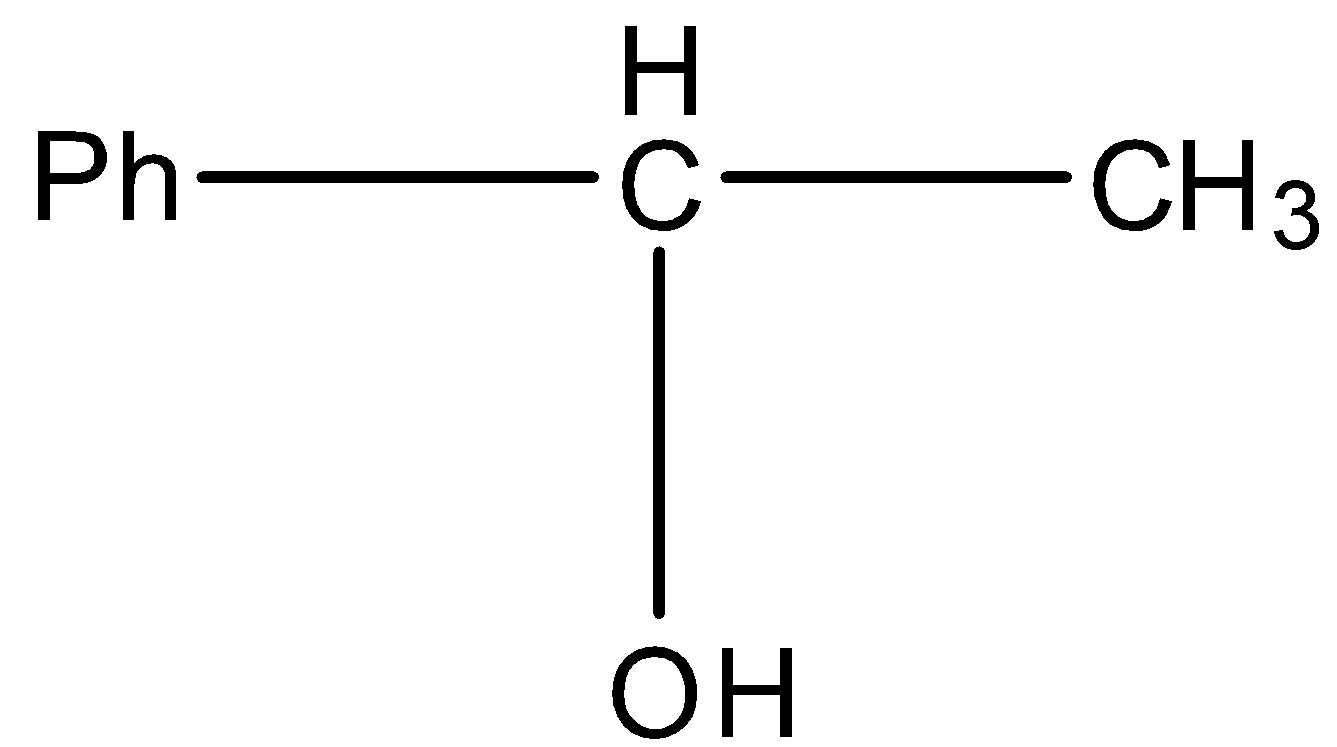
This will give the haloform reaction because there is a −COCH3 group and the −CO group is not attached to any other oxygen atom.
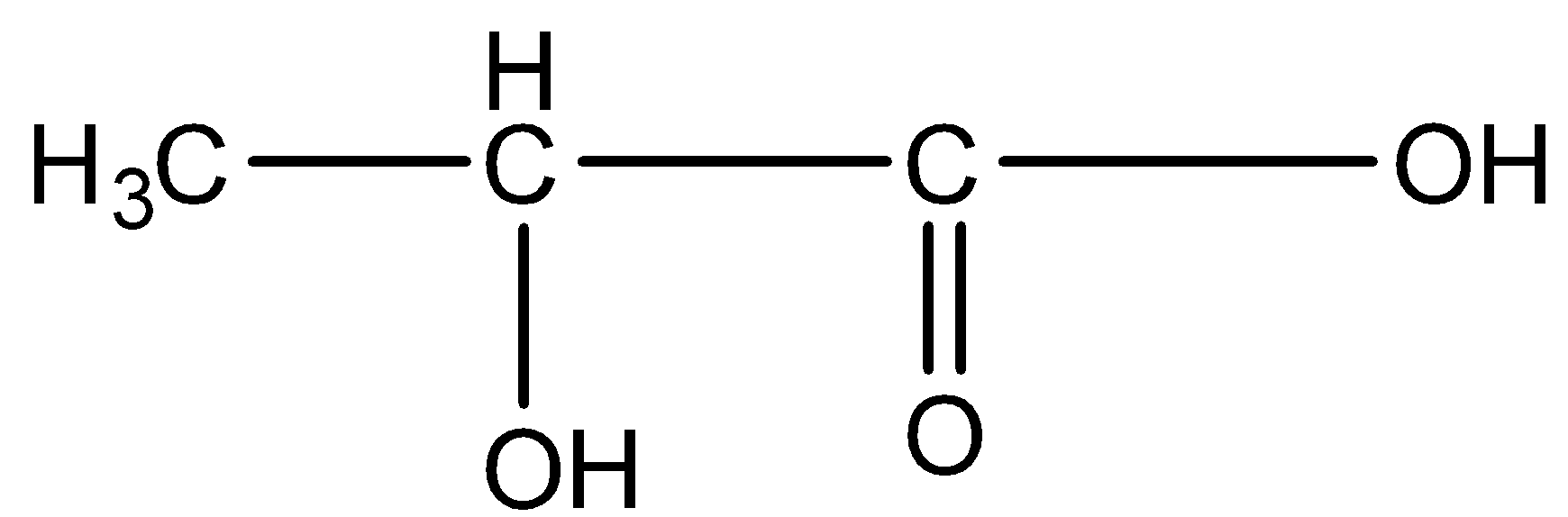
This compound will not give a haloform reaction because it doesn’t have the −COCH3 group.
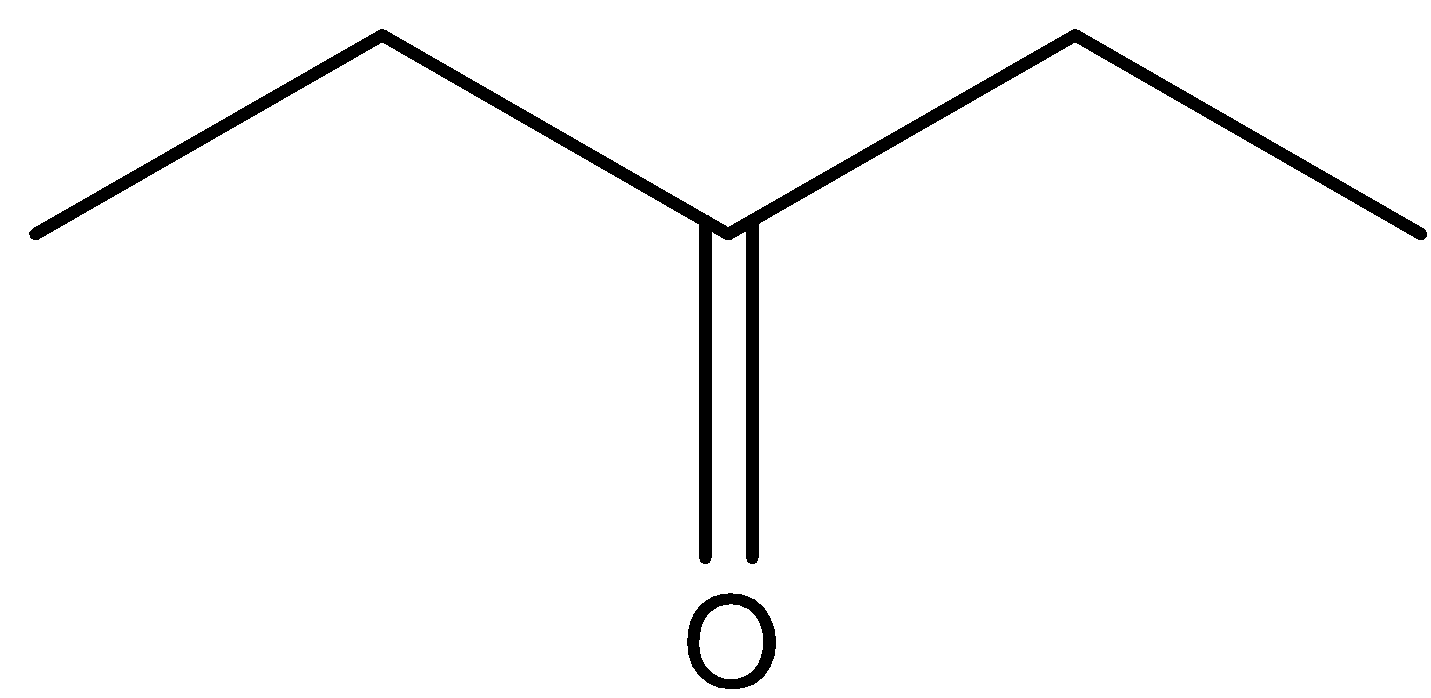
This will give the haloform reaction because there is a −COCH3 group and the −CO group is not attached to any other oxygen atom.
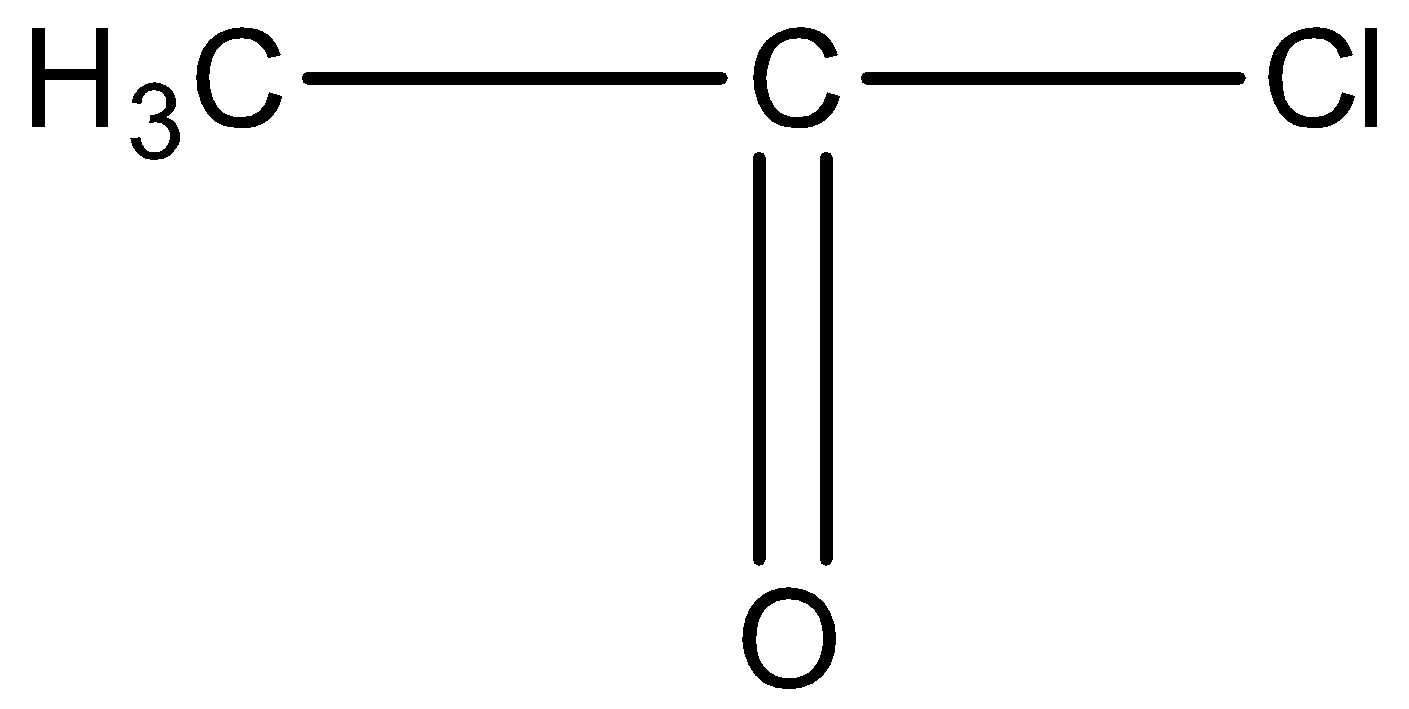
This compound will not give the haloform reaction because the −COCH3 group is attached to another oxygen atom.
So six compounds can give a haloform reaction.
Note:
In the laboratory, this reaction is very helpful in finding the presence of methyl ketone or secondary alcohol in the molecule and industrially this reaction is very helpful in making iodoform, bromoform, and chloroform.
