Question
Question: Nucleus A having Z = 17 and equal number of protons and neutrons has 1.2MeV binding energy per nucle...
Nucleus A having Z = 17 and equal number of protons and neutrons has 1.2MeV binding energy per nucleon. Another nucleus B of Z = 12 has total 26 nucleons and 1.8 MeV binding energy per nucleons. The difference of binding energy of B and A will be
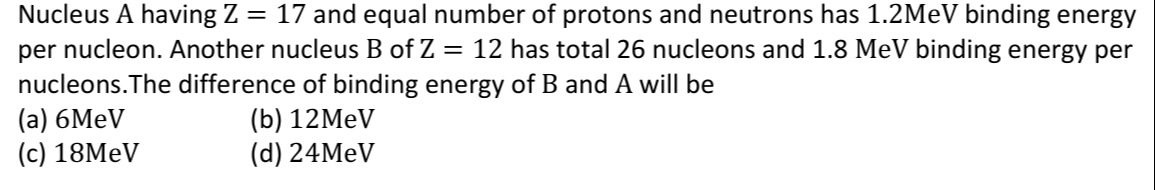
A
6MeV
B
12MeV
C
18MeV
D
24MeV
Answer
6MeV
Explanation
Solution
Here's how to calculate the difference in binding energy:
-
Calculate the total binding energy of nucleus A:
- Mass number of A (AA) = Number of protons + Number of neutrons = 17 + 17 = 34
- Total binding energy of A (BEA) = AA * (Binding energy per nucleon) = 34 * 1.2 MeV = 40.8 MeV
-
Calculate the total binding energy of nucleus B:
- Mass number of B (AB) = 26
- Total binding energy of B (BEB) = AB * (Binding energy per nucleon) = 26 * 1.8 MeV = 46.8 MeV
-
Calculate the difference in binding energy:
- Difference = BEB - BEA = 46.8 MeV - 40.8 MeV = 6.0 MeV
Therefore, the difference in binding energy between nucleus B and nucleus A is 6 MeV.
