Question
Question: No of Resonating Structures:...
No of Resonating Structures:
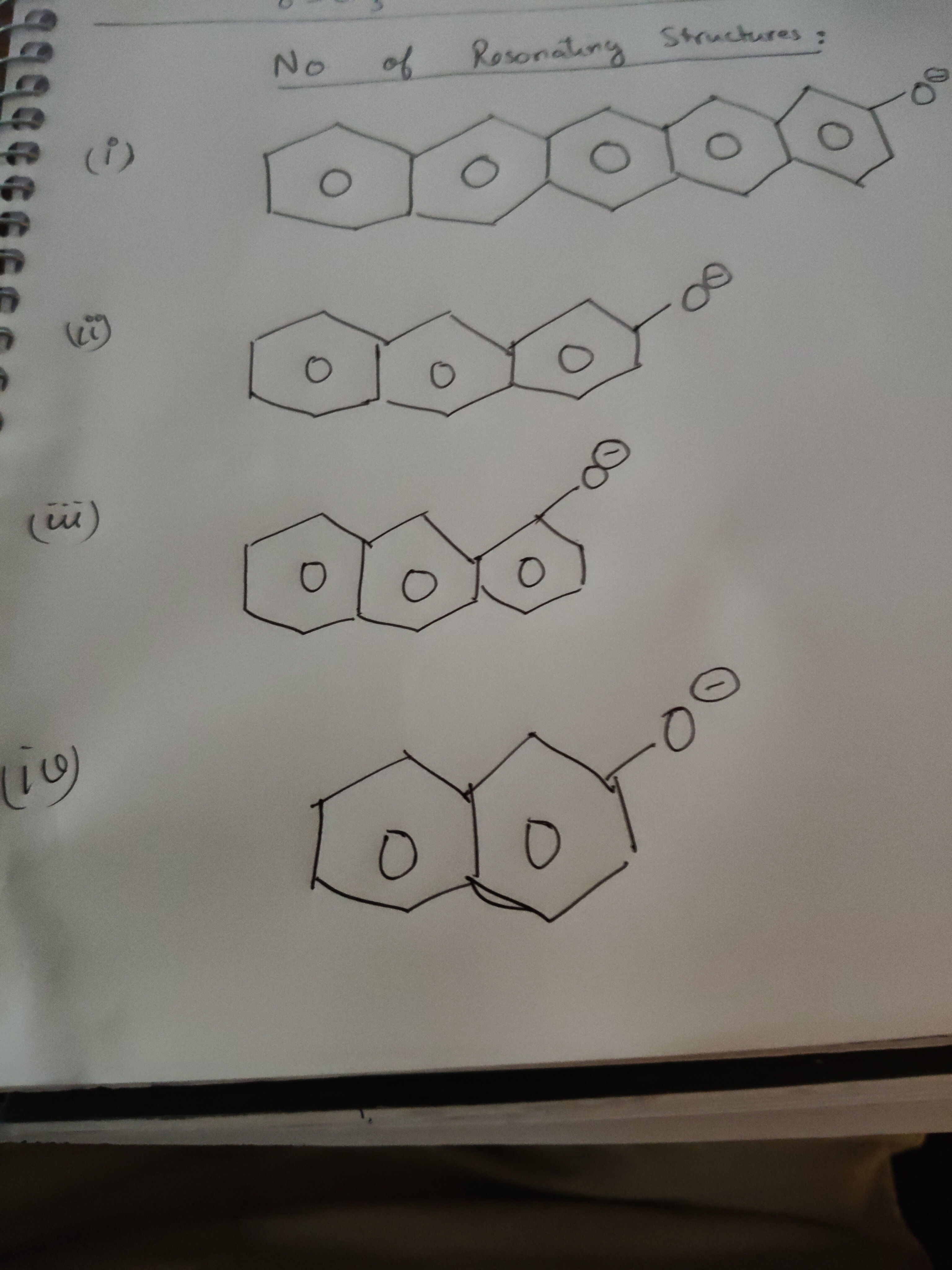
(i) 8 (ii) 6 (iii) 6 (iv) 4
Solution
To determine the number of resonance structures for each species, we analyze the delocalization of the negative charge on the oxygen atom into the conjugated pi system of the fused aromatic rings. The number of resonance structures depends on the extent of conjugation and the position of the charged group.
-
Structure (iv): This represents a naphthoxide ion. Naphthalene has 10 carbons in its conjugated system. When a negative charge is on an oxygen atom attached to an aromatic ring, it can delocalize into the ring. For naphthoxide, there are 4 unique resonance structures, with the negative charge delocalizing to the oxygen atom and three distinct carbon atoms on the naphthalene rings.
-
Structure (iii): This represents an anthracenoxide ion with the oxygen attached to a carbon in the central ring. Anthracene has 14 carbons in its conjugated system. The negative charge on oxygen can be delocalized into the pi system. For anthracenoxide, when the substituent is on a carbon of the central ring, there are 6 resonance structures.
-
Structure (ii): This represents an anthracenoxide ion with the oxygen attached to a terminal carbon of the linear fused system. For anthracenoxide with the substituent on a terminal carbon, there are 6 resonance structures.
-
Structure (i): This represents a tetracenoxide ion with the oxygen attached to a terminal carbon of the linear fused system. Tetracene has 18 carbons in its conjugated system. For tetracenoxide with the substituent on a terminal carbon, there are 8 resonance structures.
