Question
Question: No. of Resonating Structures: ...
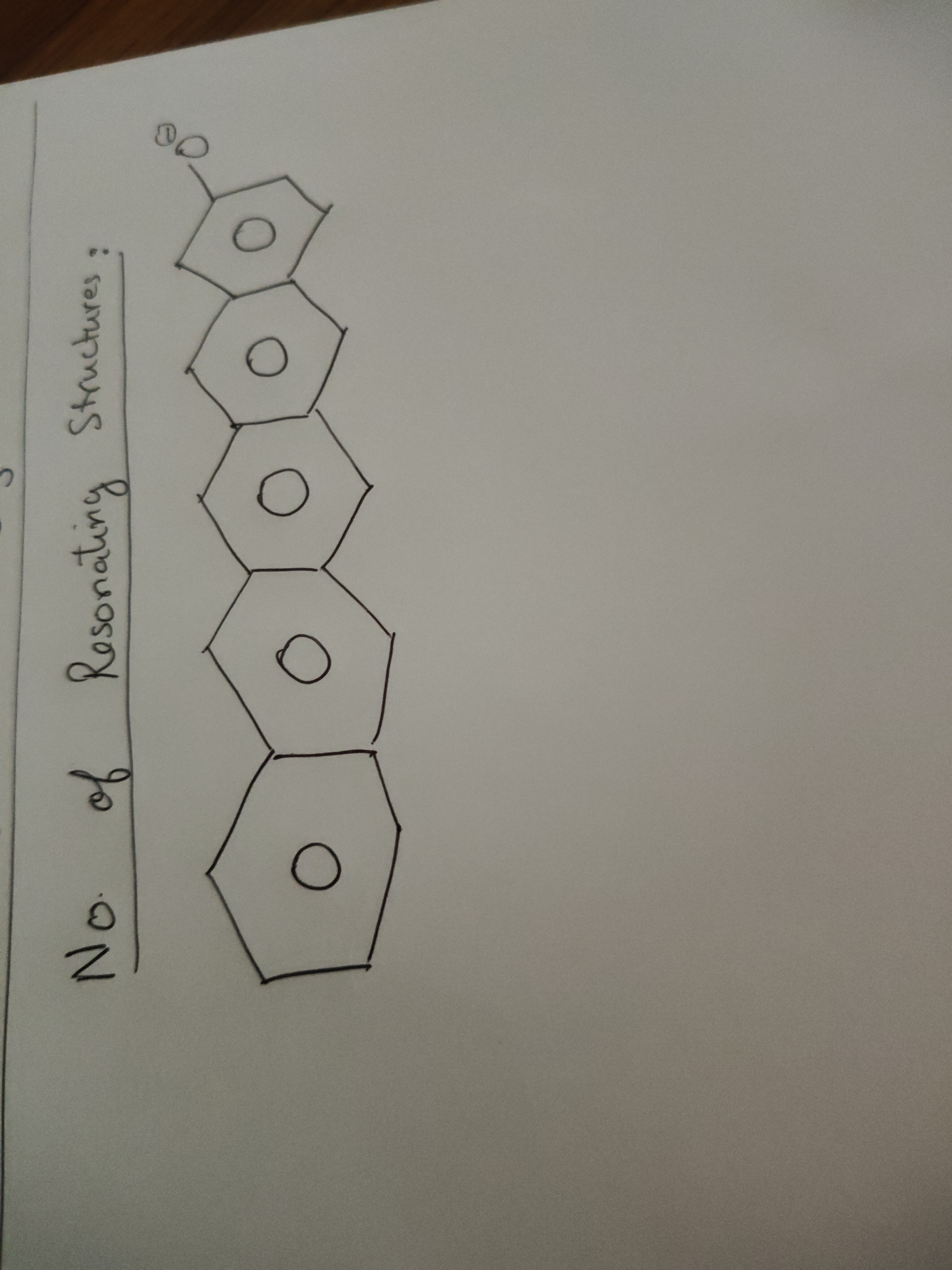
No. of Resonating Structures:
23
Solution
The given molecule is a linear polycyclic aromatic hydrocarbon (pentacene) with a negatively charged oxygen atom attached to one of its terminal positions. Resonance occurs when a charge or lone pair can be delocalized over a conjugated pi system. In this case, the negatively charged oxygen atom can donate its electron density into the pi system of pentacene.
The structure of pentacene consists of 5 linearly fused benzene rings. The number of carbon atoms in the pi system of pentacene is 22. If the oxygen atom is attached to a terminal carbon atom of pentacene, the negative charge can be delocalized to any of the carbon atoms in the pi system, as well as reside on the oxygen atom itself.
Therefore, the total number of positions where the negative charge can be located is the number of carbon atoms in the pi system of pentacene plus the oxygen atom. Number of carbon atoms in the pi system of pentacene = 22. Number of resonance structures = (Number of carbon atoms in pi system) + 1 (for the oxygen atom). Number of resonance structures = 22 + 1 = 23.
