Question
Chemistry Question on Amines
Nitration of phenyl benzoate yields the product
A

B

C

D

Answer

Explanation
Solution
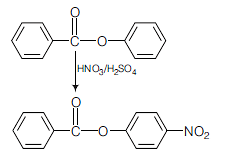
It is an electrophilic aromatic substitution reaction. Because the COO− acts as an electron withdrawing group to one phenyl group and a moderate electron donating group to the other phenyl group the nitration will occur on the phenyl group where COO− is acting as an electron donating group. The major product of the nitration will have the −NO2 in the para position because the ortho position is partially blocked by the large COPh group.
