Question
Question: Nitrating mixture is obtained by mixing of \(Conc.{\text{HN}}{{\text{O}}_3}{\text{ and Conc}}{\text{...
Nitrating mixture is obtained by mixing of Conc.HNO3 and Conc. H2SO4. role of H2SO4 in nitration is …………………….
This question has multiple correct options:
(A) To force HNO3 to behave as a base
(B) To suppress the dissociation of HNO3
(C) To produce NO+2 ions.
(D) To remove the colour NO2 produced during nitration.
Solution
Sulphuric acid is necessary as it helps in the generation of nitronium ion from nitric acid which is the actual nitrating agent. Nitronium ion is not easily produced when we use only nitric acid.
Complete answer:
Nitrating mixture is obtained by mixing of Conc. HNO3 and Conc.H2SO4
The role of H2SO4in nitrating is to produce nitronium ion by the protonation of nitric acid which causes the less water molecule and formation of nitronium ion.
In nitration of benzene
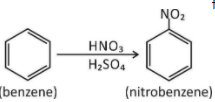
First step → Generation of electrophile in nitration of benzene is to activate HNO3with sulphuric acid to produce a stronger nucleophile, the nitronium ion.
Because the nitronium ion is a good electrophile, it is attacked by benzene to produce nitrobenzene.
Hence the correct option is A, B and C.
Note: In nitrating mixture, NO+2ion is an electrophile. It is produced by transfer of proton from sulphuric acid to nitric acid. Hence HNO3acts as a base. Nitration is used to add nitrogen to the benzene ring which can be used further in the substitution reaction. The nitro group acts as a ring deactivator. Nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term is applied to the different processes of forming nitrate esters between alcohols and nitric acid.
