Question
Question: Nickel atoms can lose two electrons to form \(Ni^{2+}\) ions. The atomic number of nickel is \(\;28\...
Nickel atoms can lose two electrons to form Ni2+ ions. The atomic number of nickel is 28 . From which orbital it can lose two electrons?
A) Ni=[Ar]183d84s2 . To form Ni2+ ion, it will lose electrons from 4s
B) Ni=[Ar]183d84s2 . To form Ni2+ ion, it will lose electrons from 3d
C) Both from 3d and 4s
D) None of the above
Solution
Nickel element belongs to the fourth period and tenth group of the periodic table. It belongs to the d -block elements also known as transition elements. The last electron entering will enter the d -orbital. To become a positive ion, the electrons that experience least attraction force from the nucleus and do not increase instability on removal, will be eliminated.
Complete answer:
Nickel is the eighth element in the first period of d -block. Hence, it contains eight electrons in the d -orbital. The atomic number of Nickel Ni is 28 . Hence, the electronic configuration is given as
Ni=1s22s22p63s23p64s23d8
Considering the configuration of the previous nearest inert gas, the electronic configuration with inert gas core can be written as
Ni=[Ar]4s23d8
Now, we can write the electron configuration of these last two orbitals with the spin of electrons as shown below;
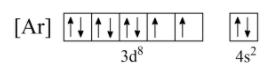
Now, in nickel, the last electron enters the d -orbital. Hence, as per the general condition, while eliminating electrons, the electrons will be removed from the d -orbital.
As per the given condition, let us assume Nickel removes two electrons from the d -orbital as shown below;
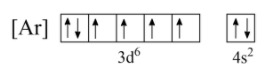
Now, from the above configuration, if electrons are eliminated from the d -orbital, it will increase the number of unpaired electrons, and as unpaired electrons are not stable, the stability of the ion Ni2+ decreases. Hence, electrons will not be removed from the 3d orbital.
Now, for the 4s orbital, even though its energy level is lower than 3d it is located farthest from the nucleus, and hence experiences least attraction from the nucleus. Hence, we can eliminate both electrons of 4s orbital as shown below;
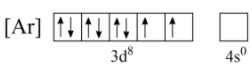
This configuration does not decrease the number of unpaired electrons, but it also does not increase it. Hence, this configuration is comparatively more stable.
Hence, Nickel will remove two electrons from 4s orbital and become Ni2+ ion.
Hence, the correct answer is Option (A)
Note: In normal state, all five d -orbitals of nickel will be degenerated i.e. at the same energy level. Hence, removing two electrons increases the number of unpaired electrons, which decreases stability. Hence, electrons will be removed from s -orbital. However, in the presence of ligands, the five orbitals do not remain degenerated, but get split into two levels. The lower energy level t2g which contains three orbitals, completely filled in nickel and the higher energy level eg which contains two orbitals, both with unpaired electrons in nickel. Hence, two remove paramagnetic effects of nickel, two electrons of s -orbital move to the d -orbital and increase stability.
