Question
Question: \({[NiC{l_4}]^{2 - }}\) show the electron filled orbitals of \(N{i^{2 + }}\) and \(C{l^ - }\) ions, ...
[NiCl4]2− show the electron filled orbitals of Ni2+ and Cl− ions, what would be the structural arrangement of the compound, octahedral or tetrahedral?
Solution
As we know that according to valence bond theory, the metal atom or ion can use (n−1)d, ns,np,nd orbitals for hybridisation under the influence of ligands to yield a set of equivalent orbitals of definite geometry like square planar, octahedral, tetrahedral and so on.
Complete step-by-step solution: We know that the Valence Bond Theory (VBT) states that the metal atom or ion use ns,np,nd and (n−1)d orbitals for the purpose of hybridisation under the influence of a ligand that results in yielding of a set of equivalent orbitals of definite geometry.
In the given complex compound, we know that one ns orbital and three np orbitals are involved in hybridisation, Nickel has a +2 oxidation state and has an electronic configuration of 3d84s0. Each chlorine ion would donate a pair of electrons to the Nickel.
We also know that the compound contain Chloride ion which are actually weak ligand, so they cannot pair up the unpaired electrons of Nickel in +2 oxidation state and we can show the hybridisation scheme of this coordination compound as:
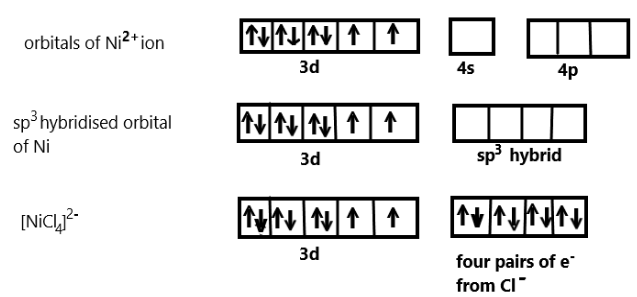
As we can see that [NiCl4]2− undergoes sp3 hybridisation to make bonds with the four chloride ion ligands thus we can say that the geometry so formed is tetrahedral. When a strong ligand like carbonyl compound is present with nickel then also the hybridisation is tetrahedral but diamagnetic as nickel contains no unpaired electron in that complex.
Therefore the correct answer is Tetrahedral geometry.
Note: Since this complex coordination compound Nickel tetrachloride possess two unpaired electrons, hence the compound is paramagnetic. Also remember when the weak ligand is present, there is no bonding between the central atom and the side atoms but when a strong ligand is present, bonding in d-orbital will take place and thus no unpaired electrons will be available which makes the compound diamagnetic.
