Question
Question: \(Ni{\left( {CO} \right)_4}\) is: (A) Tetrahedral and paramagnetic (B) Square planar and diamagn...
Ni(CO)4 is:
(A) Tetrahedral and paramagnetic
(B) Square planar and diamagnetic
(C) Tetrahedral and diamagnetic
(D) Square planar and paramagnetic
Solution
The molecular geometry of a compound shows its 3−D arrangement of an atom within a molecule. It depends upon the hybridization, whereas magnetic behavior of an atom can either be paramagnetic or diamagnetic based upon the pairing of electrons in the molecule.
Complete step by step answer:
Ni(CO)4 is nickel tetracarbonyl. It is a colourless liquid, high in toxicity.
The atomic number of Nickel (Ni) is 28 and its electronic configuration is 3d84s2
In ground state,
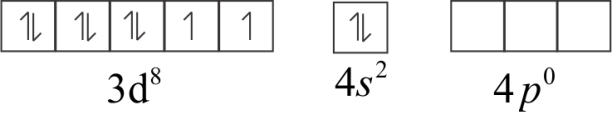
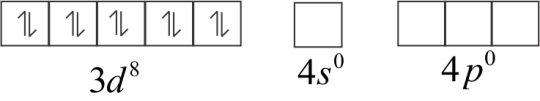
In an excited state, when CO ligand approaches it.
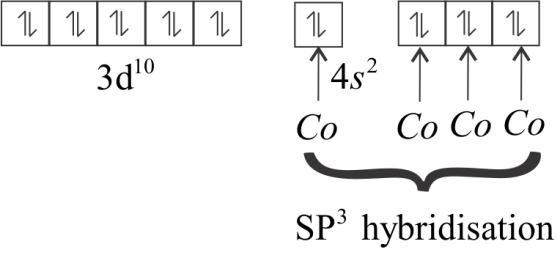
In an excited state, all the ten electrons are shifted into the 3d− orbital and get paired up.
The 4s and three 4p orbitals are empty, so they undergo sp3 hybridization. Upon hybridization, the form bonds with CO ligands and give rise to Ni(CO)4
As all the electrons in Ni(CO)4 are paired. Thus, the geometry of the molecule depends upon the hybridization. So, the geometry of Ni(CO)4 will be tetrahedral due to sp3 hybridization.
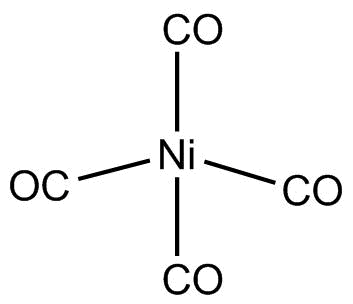
Hence, Ni(CO)4 is tetrahedral and diamagnetic in nature.
Hence, the correct option is C, tetrahedral and diamagnetic.
Additional Information:
Ludwig Mond synthesized Ni(CO)4 for the first time in 1890 the performed direct reaction of CO with Ni to obtain Ni(CO)4.
Now-a-days it is prepared in laboratories by carbonylation of bis(cyclooctadiene) nickel (O).
The vapours of Ni(CO)4 are able to auto ignite and decompose in air quickly.
Note:
Oxidation state of nickel in Ni(CO)4 can be found mathematically as:
We know the oxidation state of CO is neutral, that is zero.
Let oxidation state of Ni=x
Therefore, x+(4×0)=0
x=0
Hence, oxidation state of nickel is zero in Ni(CO)4
