Question
Question: Which is most stable alkene ?...
Which is most stable alkene ?
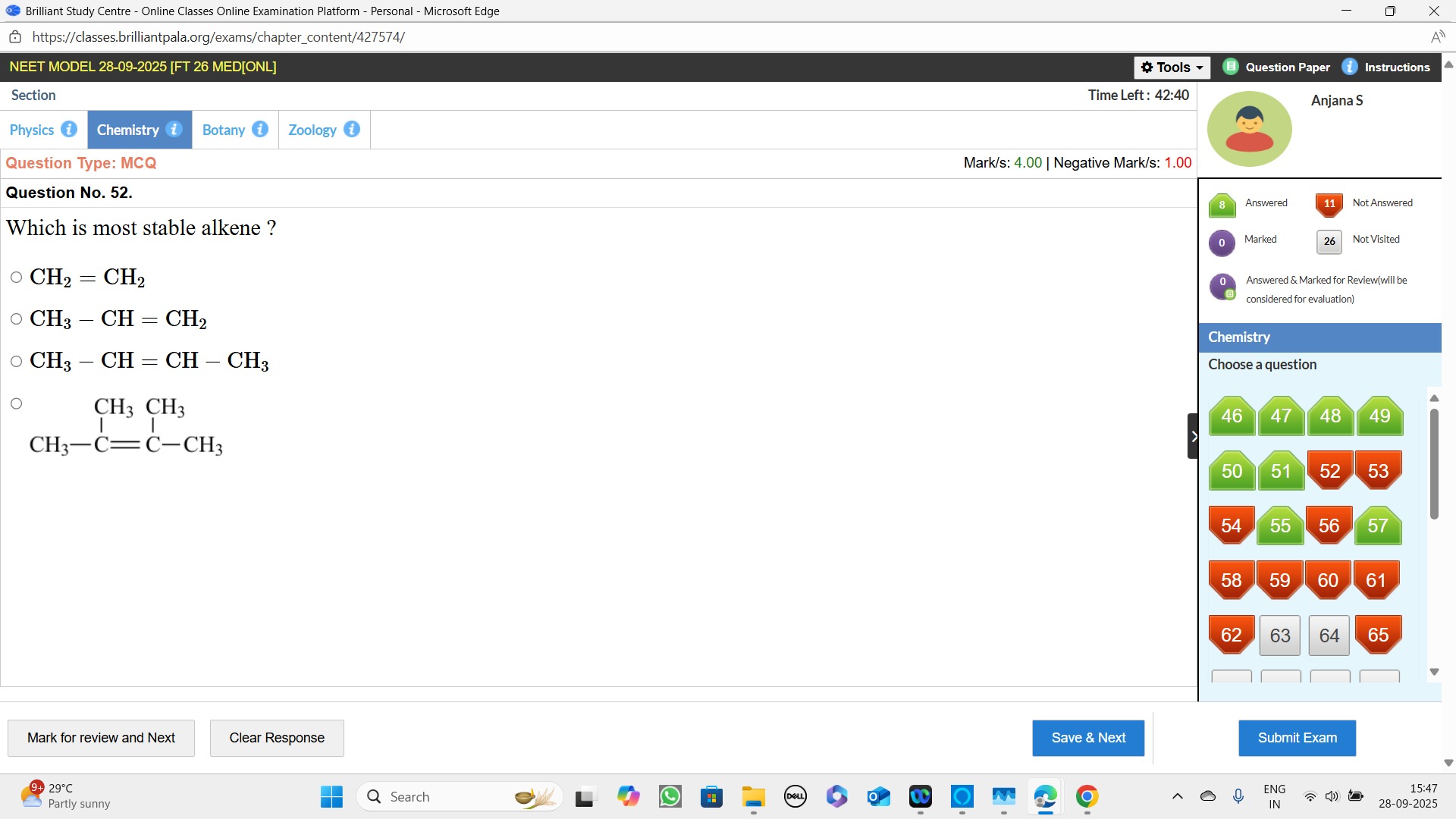
A
CH2=CH2
B
CH3−CH=CH2
C
CH3−CH=CH−CH3
D
CH3CH3CH3−C=C−CH3
Answer
CH3−C(CH3)=C(CH3)−CH3
Explanation
Solution
The stability of alkenes increases with the number of alkyl substituents attached to the double bond carbons. This is due to the electron-donating nature of alkyl groups, which stabilize the pi electron system through inductive effect and hyperconjugation.
- CH2=CH2 (Ethene) is unsubstituted (0 alkyl groups).
- CH3−CH=CH2 (Propene) is monosubstituted (1 alkyl group).
- CH3−CH=CH−CH3 (But-2-ene) is disubstituted (2 alkyl groups).
- CH3−C(CH3)=C(CH3)−CH3 (2,3-Dimethylbut-2-ene) is tetrasubstituted (4 alkyl groups). The tetrasubstituted alkene (CH3−C(CH3)=C(CH3)−CH3) has the highest degree of substitution and is therefore the most stable.
