Question
Question: For a given reaction, $\Delta$H = 35.5kJmol$^{-1}$ and $\Delta$S = 83.6JK$^{-1}$mol$^{-1}$. The reac...
For a given reaction, ΔH = 35.5kJmol−1 and ΔS = 83.6JK−1mol−1. The reaction is spontaneous at (Assume that ΔH and ΔS do not vary with temperature.)
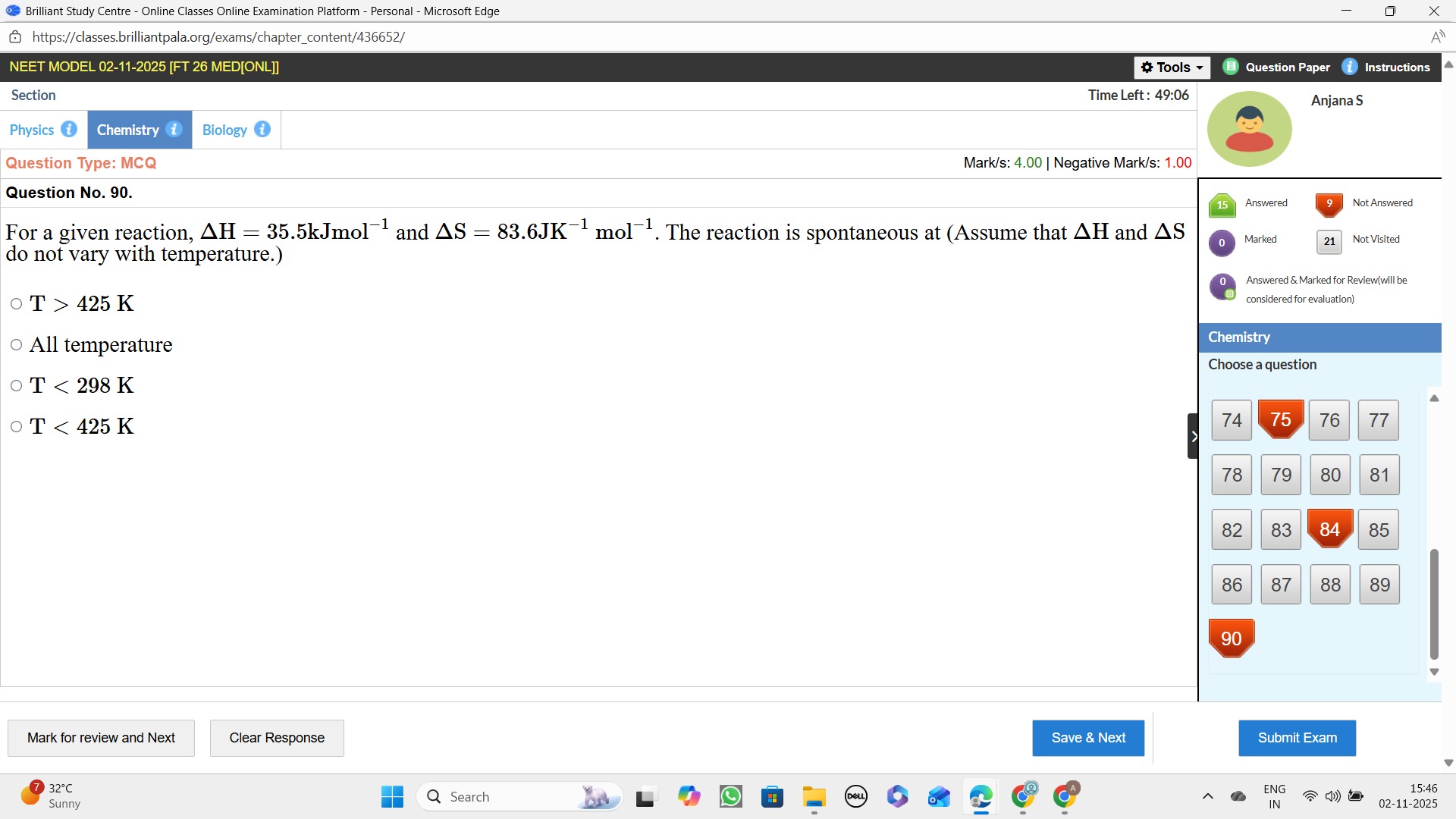
T > 425 K
All temperature
T < 298 K
T < 425 K
T > 425 K
Solution
The spontaneity of a reaction is determined by the Gibbs Free Energy change (ΔG), given by the equation ΔG = ΔH - TΔS. A reaction is spontaneous when ΔG < 0. Given ΔH = 35.5 kJmol−1 = 35500 Jmol−1 and ΔS = 83.6 JK−1mol−1. For spontaneity, ΔH - TΔS < 0, which implies ΔH < TΔS. Rearranging for temperature, T > ΔSΔH. Substituting the given values: T > 83.6 JK−1mol−135500 Jmol−1 = 424.64 K. Therefore, the reaction is spontaneous at temperatures greater than 424.64 K. Among the given options, "T > 425 K" is the condition that guarantees spontaneity.
