Question
Question: Which reaction from the following, will have maximum entropy change?...
Which reaction from the following, will have maximum entropy change?
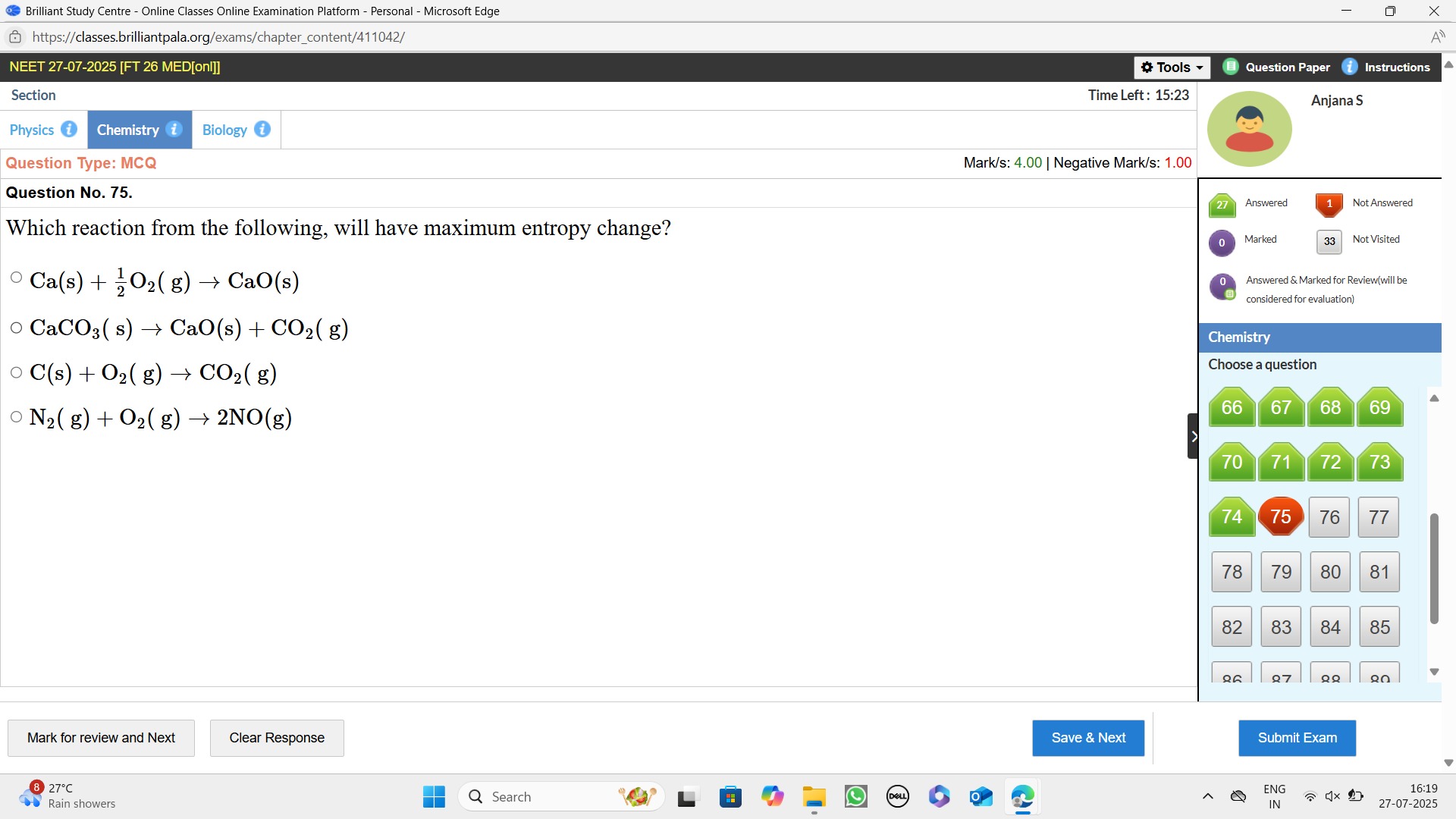
Ca(s) + 21O2(g) → CaO(s)
CaCO3(s) → CaO(s) + CO2(g)
C(s) + O2(g) → CO2(g)
N2(g) + O2(g) → 2NO(g)
CaCO3(s) → CaO(s) + CO2(g)
Solution
The entropy change (ΔS) of a reaction is primarily determined by the change in the number of moles of gaseous species (Δng). An increase in the number of gas moles leads to a positive entropy change (increase in disorder), while a decrease leads to a negative entropy change (decrease in disorder).
Let's analyze each reaction:
-
Ca(s) + 21O2(g) → CaO(s)
- Moles of gaseous reactants = 0.5
- Moles of gaseous products = 0
- Δng=0−0.5=−0.5
- Entropy decreases (ΔS<0).
-
CaCO3(s) → CaO(s) + CO2(g)
- Moles of gaseous reactants = 0
- Moles of gaseous products = 1
- Δng=1−0=+1
- Entropy increases significantly (ΔS>0).
-
C(s) + O2(g) → CO2(g)
- Moles of gaseous reactants = 1
- Moles of gaseous products = 1
- Δng=1−1=0
- Entropy change is relatively small, as the number of gas moles remains constant.
-
N2(g) + O2(g) → 2NO(g)
- Moles of gaseous reactants = 1 + 1 = 2
- Moles of gaseous products = 2
- Δng=2−2=0
- Entropy change is relatively small, as the number of gas moles remains constant.
Comparing the Δng values:
- Option 1: -0.5
- Option 2: +1
- Option 3: 0
- Option 4: 0
The reaction with the largest positive value of Δng will have the maximum positive entropy change. In this case, Option 2 has Δng=+1, which is the largest positive change.
